10 Reasons to Choose a Full Service CRO for Compliance Success

Overview
The article highlights the significant advantages of selecting a full-service Contract Research Organization (CRO) like AVS Life Sciences to enhance compliance success in clinical trials. It begins by addressing the compliance challenges faced in the industry, emphasizing the need for effective solutions.
AVS Life Sciences offers comprehensive services, expert knowledge, and proactive risk management strategies that not only improve study outcomes but also ensure adherence to regulatory standards. This combination ultimately increases the likelihood of successful approvals and facilitates efficient research processes.
By engaging with AVS Life Sciences, organizations can leverage these strengths to navigate the complexities of compliance, thereby achieving their research goals with confidence.
Introduction
Navigating the intricate landscape of clinical research can be daunting, particularly as studies increase in complexity and regulatory demands intensify. Full service Contract Research Organizations (CROs) like AVS Life Sciences provide a vital lifeline, offering a comprehensive suite of services designed to enhance compliance and streamline processes. As the stakes rise—with only a fraction of medications receiving FDA approval—organizations must consider how to leverage the full capabilities of a CRO to ensure not just survival, but success in their research endeavors.
This article explores ten compelling reasons to choose a full service CRO, revealing how such partnerships can transform challenges into opportunities for excellence in clinical trials.
AVS Life Sciences: Comprehensive Services for Clinical Trial Success
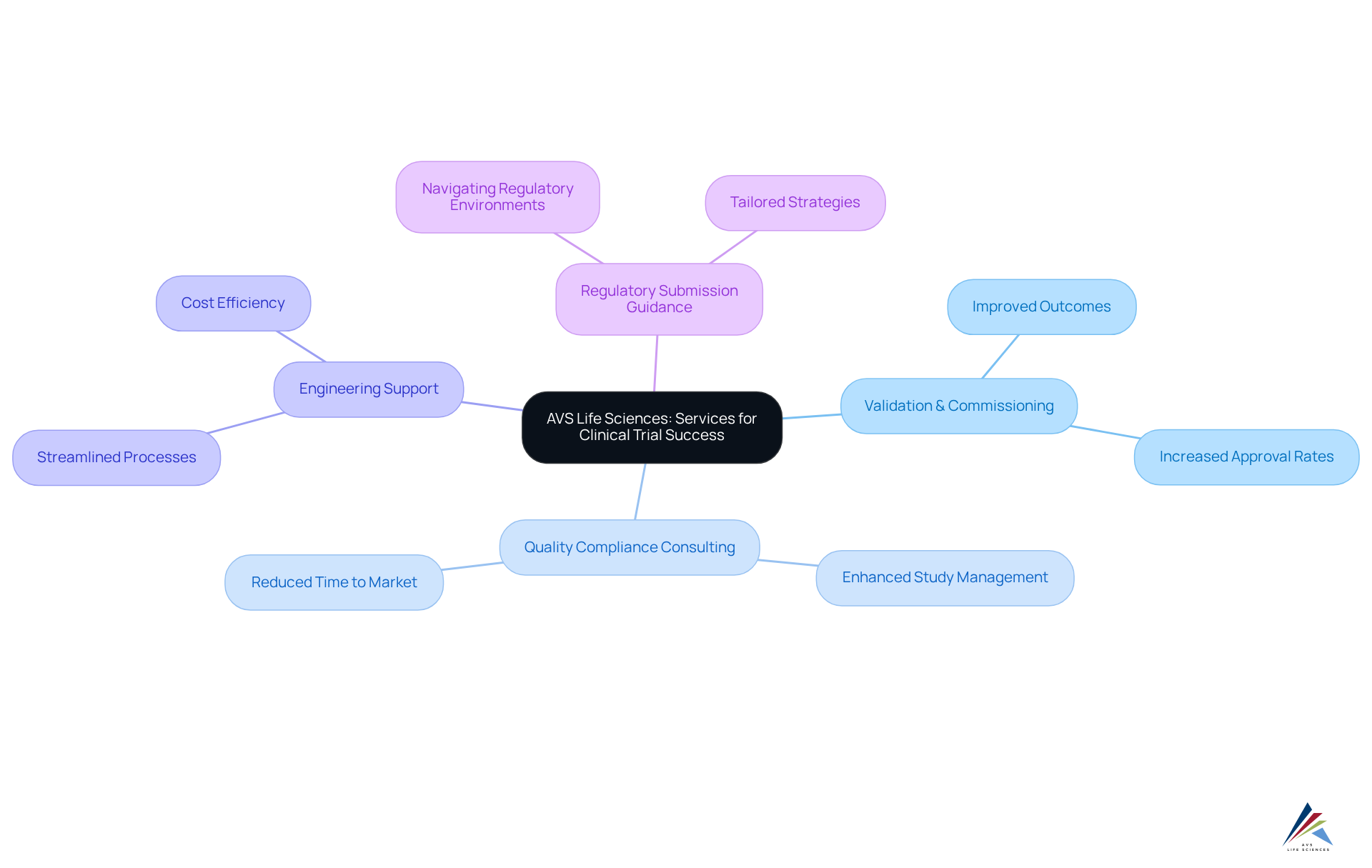
AVS Health Sciences presents a comprehensive suite of services meticulously designed for research studies, encompassing validation and commissioning, quality compliance consulting, engineering support, and guidance on regulatory submissions. This extensive service framework is crucial for delivering complete support throughout the research process, significantly enhancing the probability of success and adherence to industry standards.
With an international team of over 300 seasoned experts, AVS Life Sciences adeptly navigates the complexities of research studies, equipping clients with to effectively manage regulatory environments. Their phase-appropriate quality and regulatory strategies are carefully customized to meet the distinct requirements of each study, ensuring that every aspect of the process is executed with precision and efficiency.
The importance of comprehensive services for research success cannot be overstated. As research studies become increasingly complex—evidenced by a 61% increase in eligibility standards from 2001 to 2015—partnering with an organization that offers integrated support is paramount. Successful case studies reveal that organizations leveraging the full service CRO capabilities of AVS achieve improved outcomes, including elevated approval rates and reduced time to market.
Industry leaders emphasize the importance of full service CRO in research studies, noting that comprehensive services not only streamline processes but also enhance the overall quality of study management. By committing to thorough planning and execution, AVS Sciences positions its clients for success in a competitive landscape, ultimately contributing to the advancement of innovative therapies and improved patient outcomes. Furthermore, with only approximately 10% of medications undergoing testing receiving FDA approval, the value of AVS Life Sciences' extensive services is amplified in bolstering the chances of success.
Regulatory Compliance: Ensuring Adherence to Industry Standards
Regulatory adherence is vital in research studies, ensuring that all activities conform to established laws and guidelines, including Good Manufacturing Practices (GMP), ISO standards, and Quality System Regulations (QSR). As we approach 2025, it is anticipated that only a portion of medical studies will fully satisfy these standards, underscoring the necessity for robust compliance strategies. AVS Sciences excels in navigating this complex regulatory landscape, equipping clients with the essential tools to prepare for audits and inspections, thereby minimizing the risk of non-compliance.
Utilizing vast industry knowledge, AVS Sciences assists clients in creating that not only meet regulatory standards but also enhance the overall quality of the research process. This proactive approach is crucial, especially in light of recent updates to GMP compliance guidelines, which emphasize flexibility, ethics, quality, and the integration of digital technologies. Regulatory specialists foster a culture of compliance, where adherence to GMP is not merely a checkbox but a fundamental aspect of research integrity.
By ensuring compliance with GMP and ISO standards, alongside strong Standard Operating Procedures (SOPs) and a focus on Data Integrity Deviations, AVS Sciences aids clients in avoiding potential pitfalls, promoting an atmosphere where clinical studies are conducted ethically and responsibly. This commitment to excellence is exemplified through the successful upgrade from a Biosafety Level 1 to a Level 2 GMP facility for lentivirus production, showcasing AVS Sciences' expertise in GMP compliance, validation, and engineering solutions. The successful results of numerous experiments, where adherence to these standards has been pivotal in obtaining regulatory approval and advancing medical research, further emphasize the significance of a comprehensive compliance strategy. To enhance your compliance efforts, consider implementing regular training sessions and audits to ensure ongoing adherence to these critical standards.

Quality Management: Maintaining High Standards in Clinical Research
AVS Life Sciences places a strong emphasis on quality management throughout the clinical study process, particularly through the implementation of a comprehensive computer system validation (CSV) process. This multi-step procedure, guided by the V-Model from the Good Automated Manufacturing Practices (GAMP) 5 Guide, ensures that all experimental activities are conducted according to the . The CSV process includes critical stages such as:
- Planning
- Defining user requirement specifications (URS)
- Design specifications
- Rigorous testing phases including:
- Installation Qualification (IQ)
- Operational Qualification (OQ)
- Performance Qualification (PQ)
Each of these stages includes comprehensive documentation and quality assessments, which are crucial for upholding compliance and ensuring the dependability of results.
The firm's dedication to quality is exemplified by its impressive track record of completing projects with no findings during audits. This achievement illustrates their effectiveness in maintaining compliance and ensuring the reliability of results. By prioritizing quality management and adhering to best practices in computer system validation, AVS Life Sciences empowers clients to achieve successful outcomes while building trust with regulatory bodies and stakeholders.

Operational Efficiency: Streamlining Clinical Trial Processes
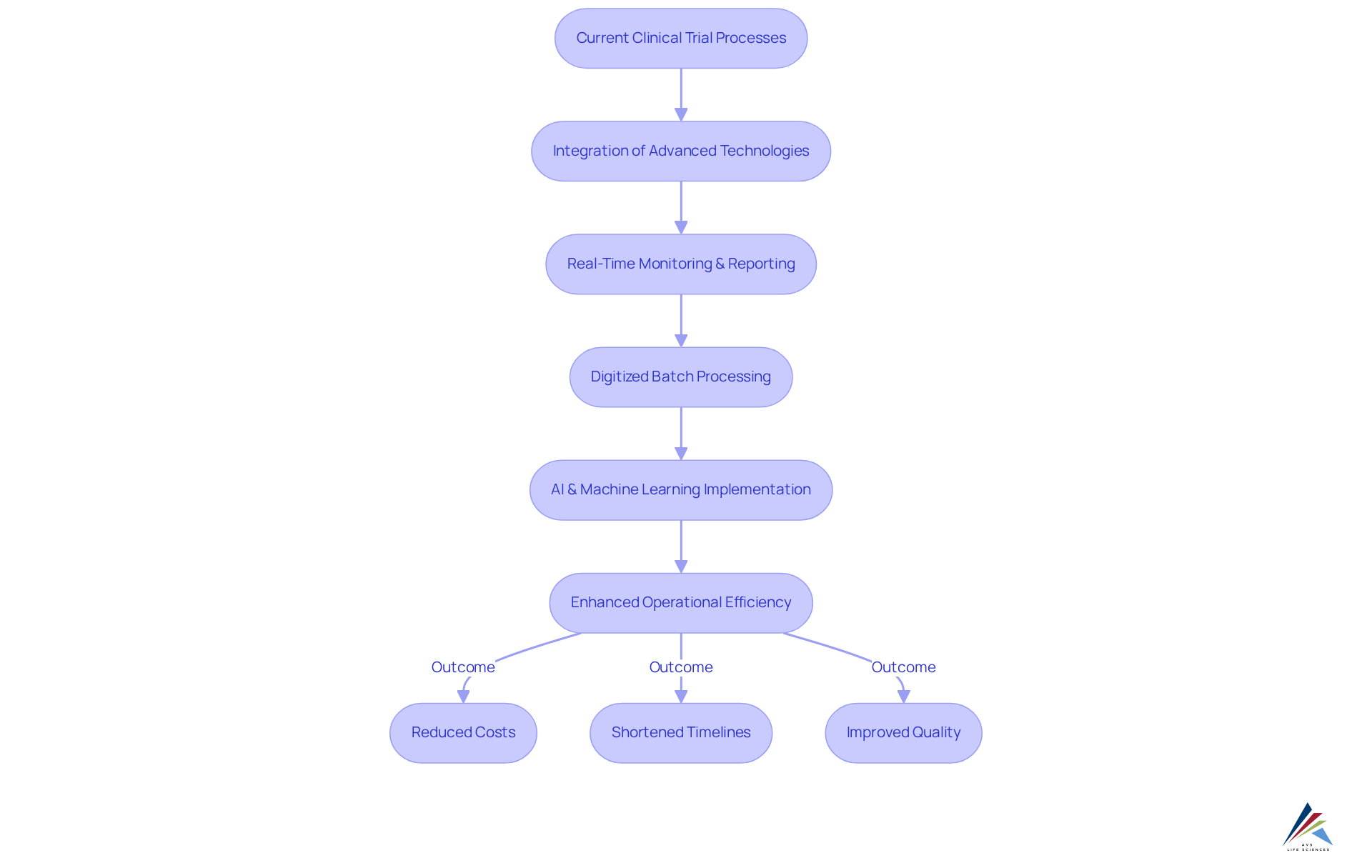
Operational efficiency stands as a cornerstone in research studies, significantly influencing both timelines and expenses. AVS Life Sciences harnesses advanced technologies and sophisticated data management systems to streamline processes, facilitating real-time monitoring and reporting. This strategic approach not only enhances workflows but also minimizes redundancies, resulting in quicker and more .
A compelling illustration of AVS's impact is evident in the integration of digitized batch processing solutions for a prominent pharmaceutical client. By linking numerous PLCs and HMIs to a site-wide industrial network, AVS improved data accessibility and operational efficiency. This integration enabled timely updates and enhanced access management, allowing operators to concentrate on vaccine production rather than IT challenges. The implementation of a PI data historian expedited deviation investigations, significantly curtailing costs associated with discarded batches, which previously surpassed $1 million each, while also saving valuable time in identifying and resolving deviations.
Statistics indicate that employing AI and machine learning techniques can compress development timelines by an average of six months per asset, translating into substantial cost savings. Furthermore, generative AI has shown the capacity to reduce study report drafting timelines by as much as 40 percent, shortening the process from weeks to days. Such efficiencies not only accelerate execution but also elevate the overall quality of outcomes, ensuring projects are completed on schedule and within budget.
Industry leaders underscore the necessity of these advancements. As Dr. Stephen Agboola noted, expanding remote monitoring initiatives may empower patients to build self-competence and enhance results, underscoring the importance of sustained patient involvement in research studies. Additionally, Anton Mihic articulated that Gen AI is poised to revolutionize research studies, refining processes to be more efficient and aligned with stakeholder requirements. By focusing on operational efficiency, AVS Life Sciences empowers clients to navigate the complexities of development with confidence, ultimately fostering improved health outcomes.
Logistical Expertise: Navigating Complex Clinical Trial Requirements
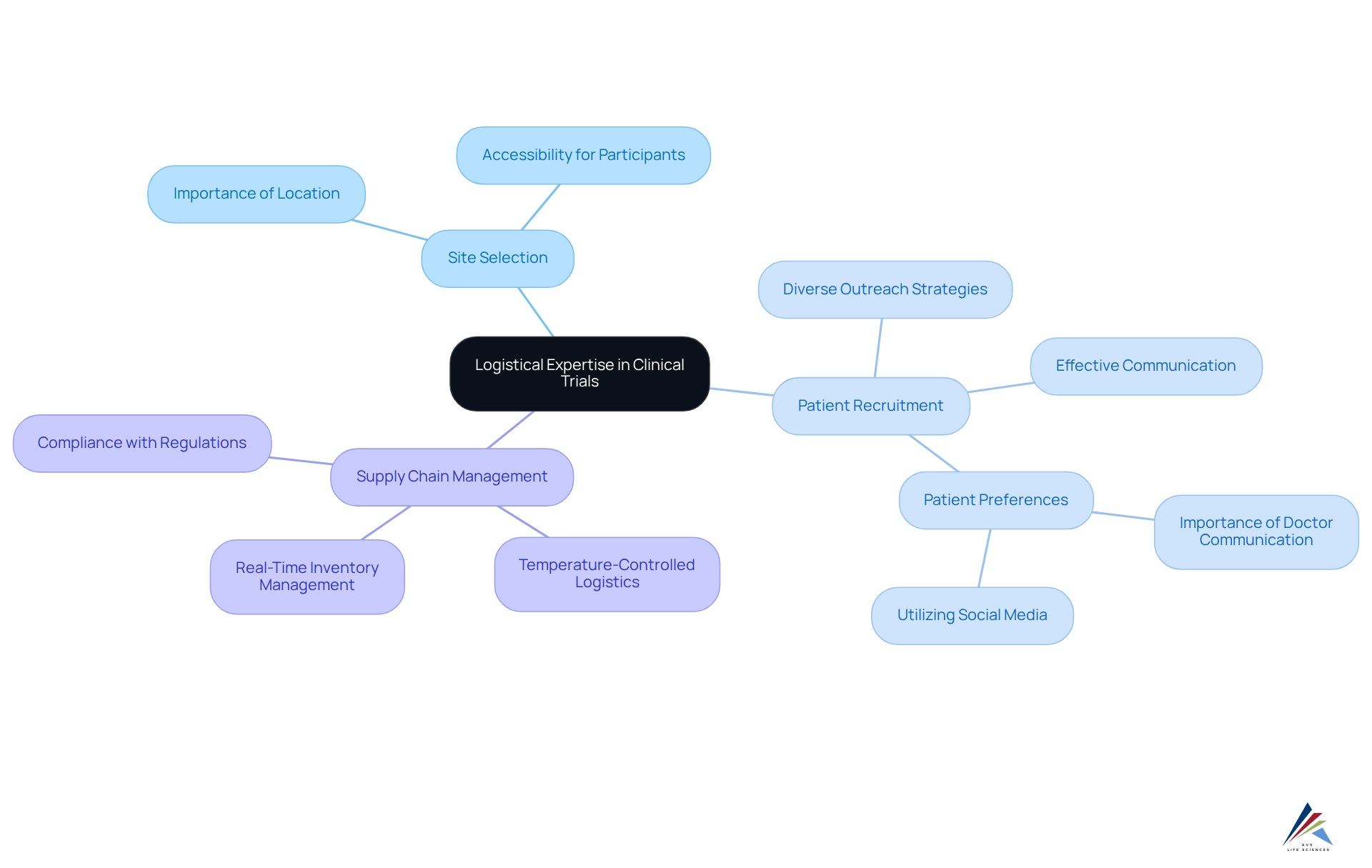
Navigating the logistical complexities of clinical studies presents significant challenges that can profoundly influence timelines and outcomes. AVS Health Sciences excels as a full service CRO, delivering comprehensive logistical support that encompasses:
- Site selection
- Patient recruitment
- Supply chain management
This ensures seamless integration of all study components.
With a strong emphasis on , AVS Life Sciences empowers clients to concentrate on the scientific elements of their studies while adeptly managing the logistical details. This strategic approach not only mitigates delays but also enhances overall efficiency, leading to improved success rates. Indeed, studies reveal that logistical barriers contribute to over 80% of medical studies facing delays or closures due to recruitment issues. By leveraging their extensive knowledge, AVS Sciences aids clients in navigating these challenges effectively, ultimately fostering more successful study outcomes.
Data Management: Ensuring Accurate and Reliable Trial Results
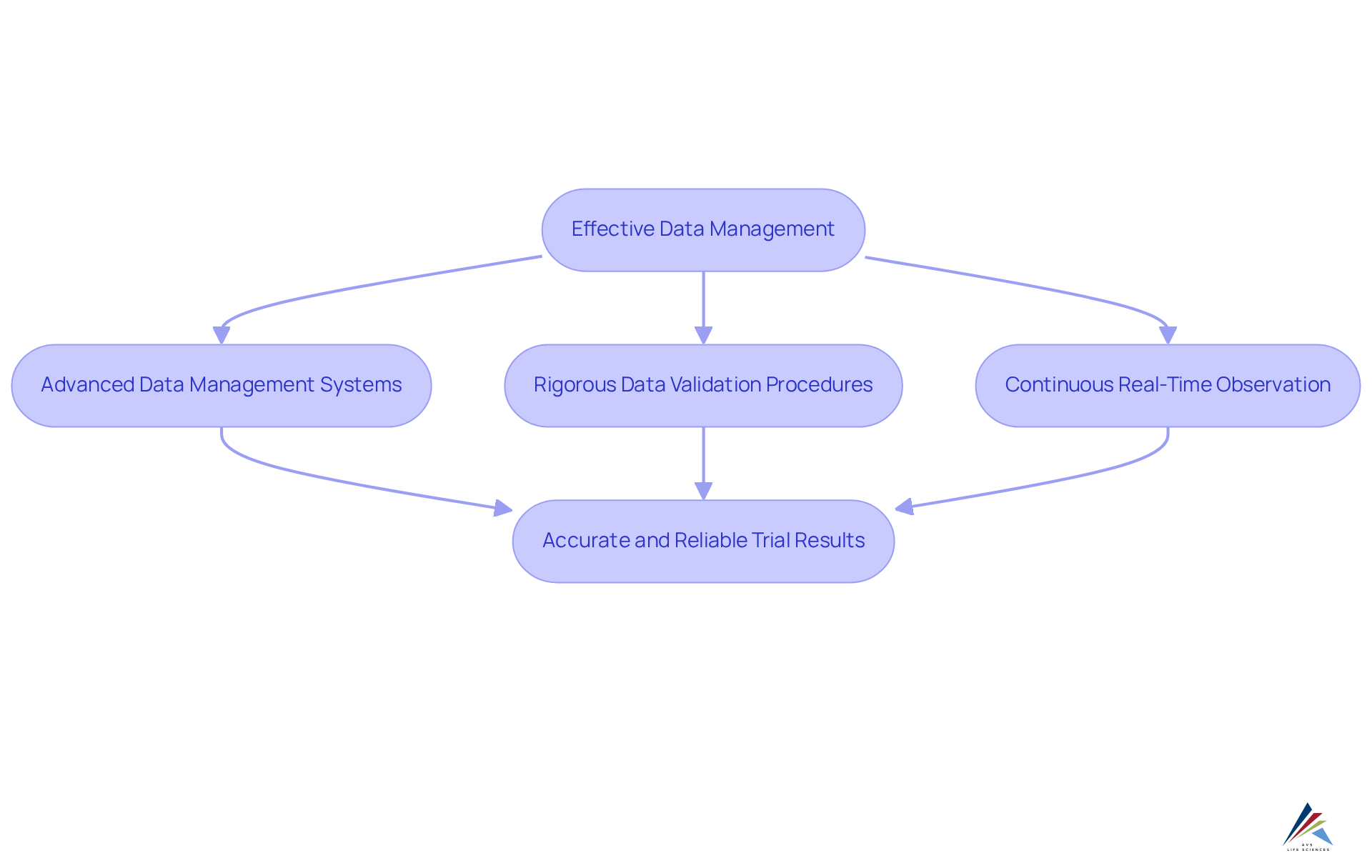
Effective data management is paramount for ensuring the accuracy and reliability of clinical research results. AVS Health Sciences employs advanced data management systems to meticulously gather, analyze, and present study data, guaranteeing that all information remains secure and adheres to regulatory standards.
By implementing rigorous data validation procedures and continuous real-time observation, AVS Health Sciences significantly enhances the integrity of study data. This method is crucial for informed decision-making and securing regulatory approval. As emphasized by industry experts, maintaining high data integrity is foundational for trustworthy study outcomes, fostering confidence among stakeholders and regulatory bodies alike. The commitment to not only facilitates compliance with regulations but also establishes AVS Health Sciences as a full service CRO that delivers precise and actionable research outcomes.
Specialized Knowledge: Leveraging Expertise for Better Outcomes
AVS Healthcare proudly presents a team of experts, including Nykkytta Mendez, an Account Manager with specialized knowledge across various research domains such as regulatory compliance, quality assurance, and data management. This depth of expertise empowers them to deliver tailored solutions that effectively address the unique challenges faced by each client.
By leveraging their extensive industry knowledge, AVS Life Sciences aids clients in navigating complex regulatory landscapes and implementing best practices that enhance study outcomes. Their expert insights not only streamline the efficiency of studies but also significantly increase the likelihood of favorable results and regulatory approval.
Research indicates that effective project management and collaboration are vital in the research process, exemplified by the FDA conducting nearly 72,000 inspections from FY 2019 through FY 2024. This underscores the critical importance of , as emphasized by AVS Life Sciences, which enhances the credibility of evaluation reports and facilitates successful market access.
As we look towards 2025, the evolution of medical trials into more personalized and technology-driven healthcare highlights the growing recognition of expert consulting as a key factor in improving trial outcomes. Industry leaders assert that harnessing specialized knowledge can lead to more efficient processes and improved compliance, ultimately fostering a culture of excellence in research. AVS Health Sciences stands as a testament to this commitment to quality and success, ensuring that clients are thoroughly equipped to navigate the demands of an ever-evolving regulatory landscape.
Collaborative Partnerships: Enhancing Trial Success Through Teamwork
AVS Life Sciences recognizes that the success of clinical studies hinges on robust collaborative partnerships among all stakeholders. By prioritizing teamwork, the company cultivates open communication and collaboration among clients, regulatory bodies, and research teams. This strategic approach not only enhances but also ensures alignment of goals among all parties involved.
Successful collaborations yield improved efficiency in the testing process, as evidenced by research indicating that strong communication significantly boosts the likelihood of success. In fact, approximately 80% of research studies fail to meet initial enrollment targets, resulting in substantial financial losses—estimated at $8 million daily for pharmaceutical development firms. By nurturing a culture of trust and openness, AVS Life Sciences adeptly navigates the complexities of medical research, ultimately driving superior outcomes for all stakeholders.
Industry leaders stress that teamwork is critical for achieving shared objectives. A seasoned pharmaceutical strategist aptly notes that the collective strength of a team can generate innovative solutions that surpass individual contributions. This cooperative spirit is not merely a tactic; it is a fundamental principle that underpins AVS Life Sciences' operations, ensuring that every study is poised for success.
Scalability: Adapting Services to Meet Trial Needs
AVS Health Sciences offers adaptable solutions meticulously tailored to the unique requirements of each research study. Whether collaborating with emerging startups or established Fortune 100 firms, the company proficiently adjusts its services to offer a that provides comprehensive support throughout every stage of the testing process.
This adaptability empowers AVS Health Sciences to swiftly respond to evolving demands and challenges, enabling clients to effectively navigate the complexities of research while maintaining rigorous standards of quality and compliance. By strategically broadening their service offerings, AVS Health Sciences significantly enhances the overall effectiveness and success rates of research studies, solidifying its position as a trusted partner in the biosciences sector.
As Jack Butcher aptly states, "Skill before scale," underscoring the necessity of a robust foundation prior to service expansion. This principle is clearly reflected in AVS Health Sciences' methodology, where they prioritize quality and compliance while remaining responsive to client needs.
Statistics indicate that the full service CRO market in healthcare is projected to grow substantially, reaching USD 80.61 billion by 2030, highlighting the critical importance of flexibility in clinical research services. AVS Health Sciences exemplifies this agility through successful case studies, including their partnership with a leading biopharmaceutical company, where they utilized full service CRO capabilities to address specific study requirements, resulting in a remarkable 30% increase in efficiency.
In light of recent trends for 2025, AVS Health Sciences is committed to integrating advanced technologies and methodologies to enhance research processes. For pharmaceutical compliance officers, it is vital to recognize how flexible services can lead to improved outcomes in medical studies. By leveraging the expertise of AVS Health Sciences, organizations can ensure they remain at the forefront of compliance and quality in an ever-evolving landscape.
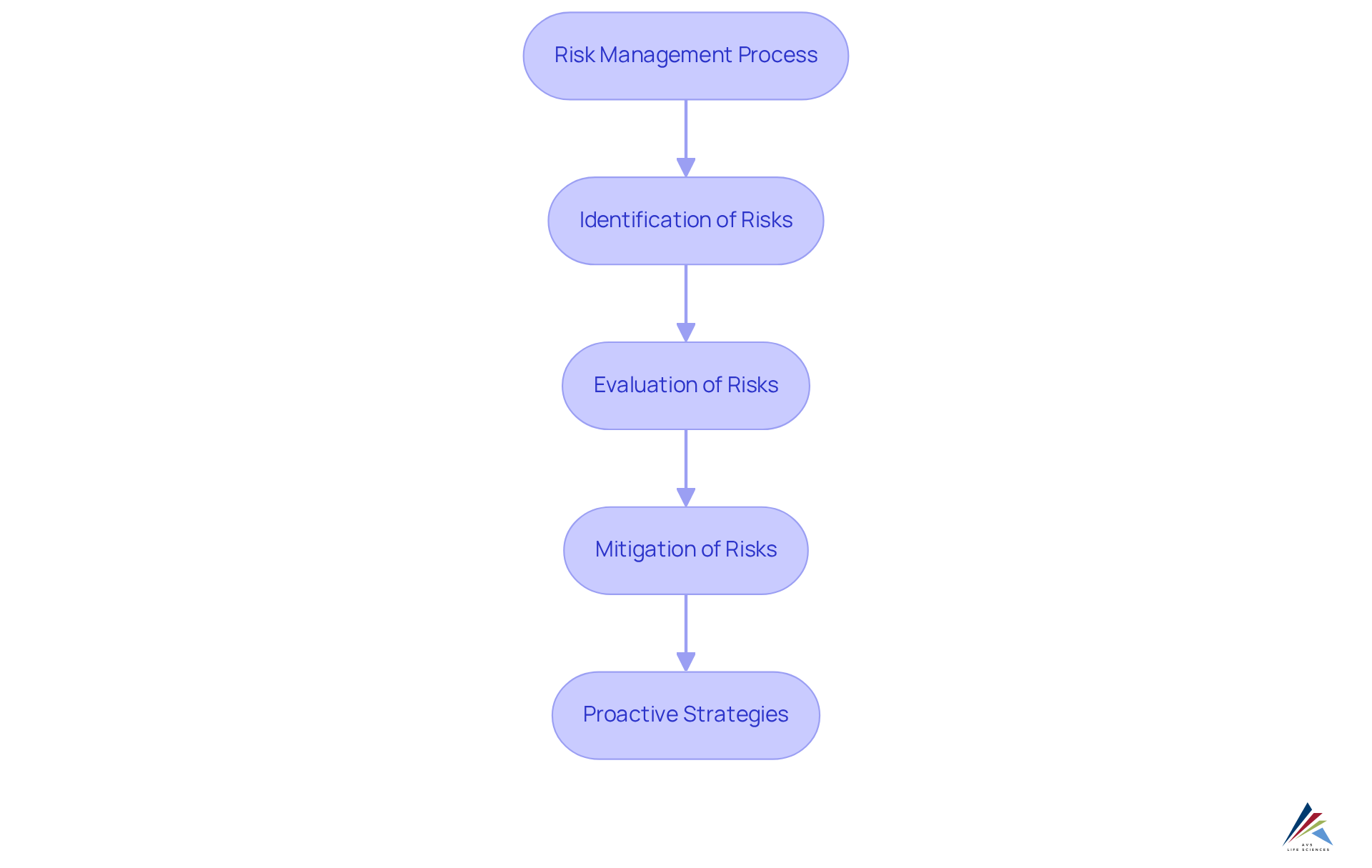
Risk Management: Proactively Addressing Challenges in Clinical Trials
Efficient risk management is paramount in research studies, encompassing the identification, evaluation, and mitigation of potential obstacles that could jeopardize results. AVS Health Sciences employs a proactive strategy, leveraging comprehensive risk assessment frameworks to pinpoint issues early in the testing process. By implementing robust risk reduction strategies, AVS Life Sciences equips clients to confront challenges as they emerge, thereby minimizing disruptions and enhancing the overall success of clinical studies. This dedication to not only protects client investments but also fosters confidence among stakeholders and regulatory authorities.
Recent updates in 2025 reveal that nearly 77% of ongoing studies integrate at least one element of Risk-Based Monitoring (RBM), highlighting the industry's transition towards proactive methodologies. Experts emphasize that early evaluation of design risks enables sponsors to address potential challenges before they adversely affect study outcomes. Such insights bolster the effectiveness of AVS Life Sciences' approaches, which are meticulously aligned with the trial's specific objectives and endpoints, ensuring a concentrated focus on the most critical risks.
Conclusion
AVS Life Sciences emphasizes the pivotal role that full-service Contract Research Organizations (CROs) have in ensuring compliance success within clinical trials. By providing a comprehensive suite of services—from regulatory compliance and quality management to operational efficiency and risk management—AVS Life Sciences establishes itself as an indispensable partner for organizations navigating the intricacies of research studies. This strategic approach not only increases the likelihood of favorable outcomes but also guarantees adherence to industry standards, ultimately fostering innovation in medical research.
The article presents several compelling arguments advocating for the selection of a full-service CRO. Key points include:
- The necessity of specialized knowledge in regulatory compliance
- The critical importance of maintaining high-quality standards throughout the clinical research process
- The value of collaborative partnerships that enhance effective communication among stakeholders
Furthermore, the focus on operational efficiency and proactive risk management illustrates how AVS Life Sciences utilizes advanced technologies and methodologies to streamline processes and mitigate potential challenges, thereby improving study success rates.
In conclusion, the importance of partnering with a full-service CRO like AVS Life Sciences cannot be overstated. As the landscape of clinical research continues to evolve, organizations must acknowledge the advantages of comprehensive support in ensuring regulatory compliance and achieving successful study outcomes. By leveraging the services offered by AVS Life Sciences, clients can navigate the complexities of clinical trials with confidence and contribute to the advancement of innovative therapies that enhance patient outcomes. The future of medical research relies on such collaborative efforts, rendering the choice of a full-service CRO a strategic necessity for success.
Frequently Asked Questions
What services does AVS Life Sciences offer for clinical trials?
AVS Life Sciences provides a comprehensive suite of services including validation and commissioning, quality compliance consulting, engineering support, and guidance on regulatory submissions to enhance research study success.
How does AVS Life Sciences support regulatory compliance?
AVS Life Sciences helps clients navigate the regulatory landscape by equipping them with tools to prepare for audits and inspections, creating compliance structures that meet regulatory standards, and fostering a culture of compliance focused on Good Manufacturing Practices (GMP) and ISO standards.
Why is comprehensive service important in research studies?
Comprehensive services are crucial as research studies have become more complex, with increased eligibility standards. Partnering with a full-service organization like AVS can lead to improved outcomes, elevated approval rates, and reduced time to market.
What is the significance of quality management in clinical research?
Quality management ensures that all activities in clinical studies are conducted to the highest standards, maintaining compliance and the reliability of results. AVS Life Sciences implements a thorough computer system validation (CSV) process to uphold these standards.
What is the computer system validation (CSV) process used by AVS Life Sciences?
The CSV process includes several critical stages: planning, defining user requirement specifications (URS), design specifications, and rigorous testing phases such as Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ), all supported by comprehensive documentation.
How does AVS Life Sciences ensure the quality of its services?
AVS Life Sciences has a strong track record of completing projects with no findings during audits, demonstrating their effectiveness in maintaining compliance and ensuring reliable results through rigorous quality management practices.
What challenges do medical studies face regarding regulatory compliance?
It is anticipated that only a portion of medical studies will fully satisfy regulatory standards by 2025, highlighting the need for robust compliance strategies to minimize the risk of non-compliance.
How does AVS Life Sciences contribute to the advancement of innovative therapies?
By providing comprehensive support and ensuring regulatory compliance, AVS Life Sciences positions its clients for success in a competitive landscape, ultimately contributing to the development of innovative therapies and improved patient outcomes.
