10 Top Pharma Consulting Firms for Compliance Excellence

Overview
This article identifies the leading pharmaceutical consulting firms that excel in compliance services, addressing the critical challenges faced by organizations in navigating complex regulatory environments. Among these firms, AVS Life Sciences stands out for its expertise and comprehensive solutions, which significantly enhance operational efficiency and uphold high compliance standards. Through successful case studies, AVS Life Sciences demonstrates its commitment to quality management, showcasing its ability to guide clients through intricate regulatory landscapes. By engaging with AVS Life Sciences, organizations can effectively address compliance challenges and achieve sustainable success.
Introduction
The pharmaceutical industry is facing an increasingly complex regulatory environment, making compliance more critical than ever. As companies strive for operational excellence, consulting firms play a paramount role, delivering the expertise necessary to meet stringent standards and drive innovation. Yet, with numerous firms claiming to offer the best solutions, organizations must discern the top players that genuinely excel in compliance and quality assurance. This article explores the ten leading pharma consulting firms recognized for their unwavering commitment to compliance excellence, equipping readers with valuable insights to forge informed partnerships in the ever-evolving landscape of life sciences.
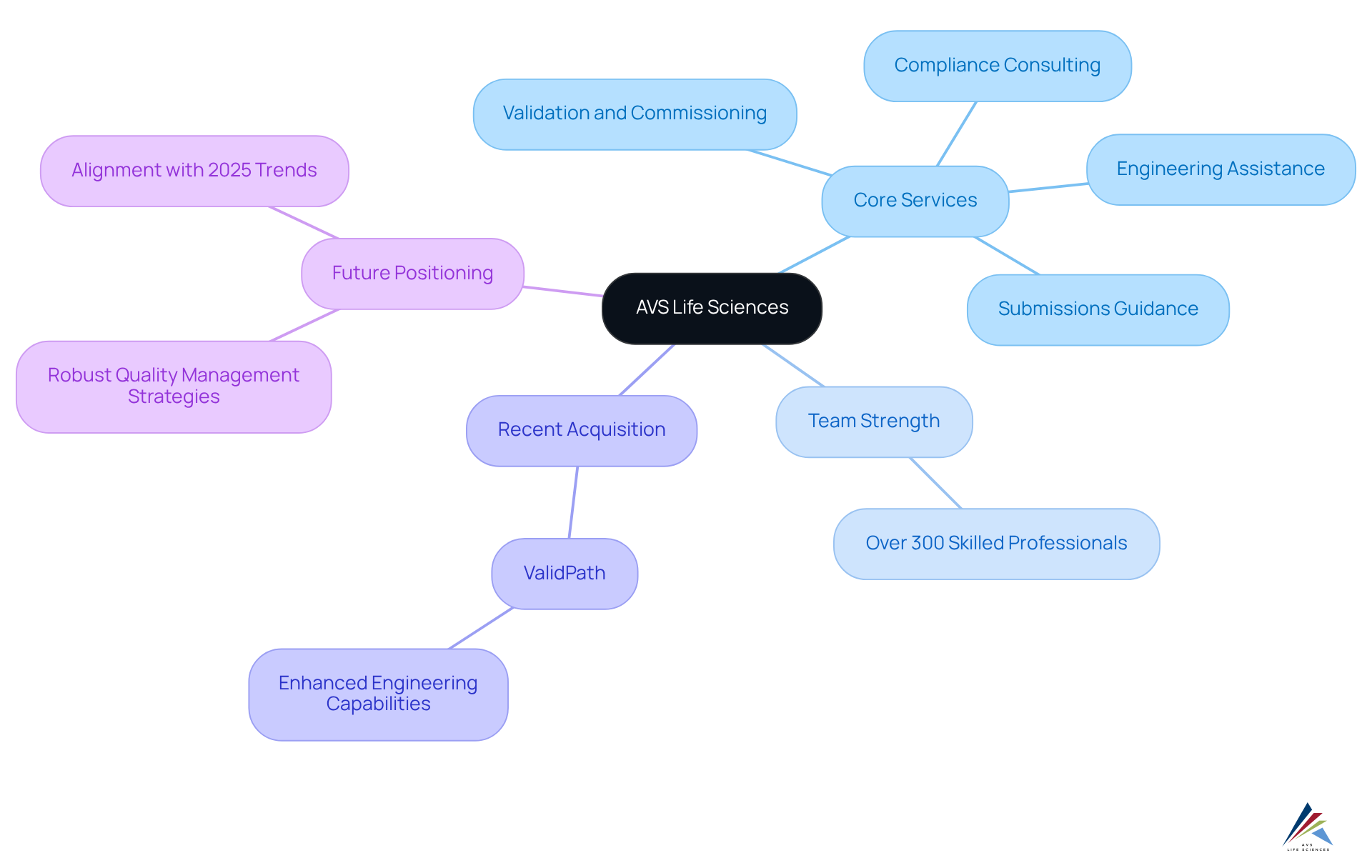
AVS Life Sciences: Comprehensive Regulatory and Quality Solutions for Life Sciences
AVS Life Sciences distinguishes itself as a leading service provider committed to delivering superior management solutions, regulatory compliance, engineering, automation, validation, and clinical services specifically designed for the pharmaceutical, biotechnology, medical device, and diagnostic sectors. By empowering clients throughout the life science product lifecycle, AVS facilitates effective navigation through complex compliance landscapes while upholding rigorous standards.
Their core services encompass:
- Validation and commissioning
- Compliance consulting
- Engineering assistance
- Submissions guidance
All executed by a dedicated team of over 300 skilled professionals worldwide. This unwavering commitment to innovation and expertise has cemented AVS's standing as a trusted partner in the life sciences domain, evidenced by an impressive 80% repeat business rate.
The recent acquisition of ValidPath enhances AVS's engineering capabilities, positioning the company to adeptly meet the industry's evolving demands. As the pharmaceutical landscape continues to transform, AVS Life Sciences remains at the forefront, implementing robust quality management strategies that align with the latest trends and compliance expectations for 2025.
IQVIA: Data-Driven Insights and Consulting for Pharma Success
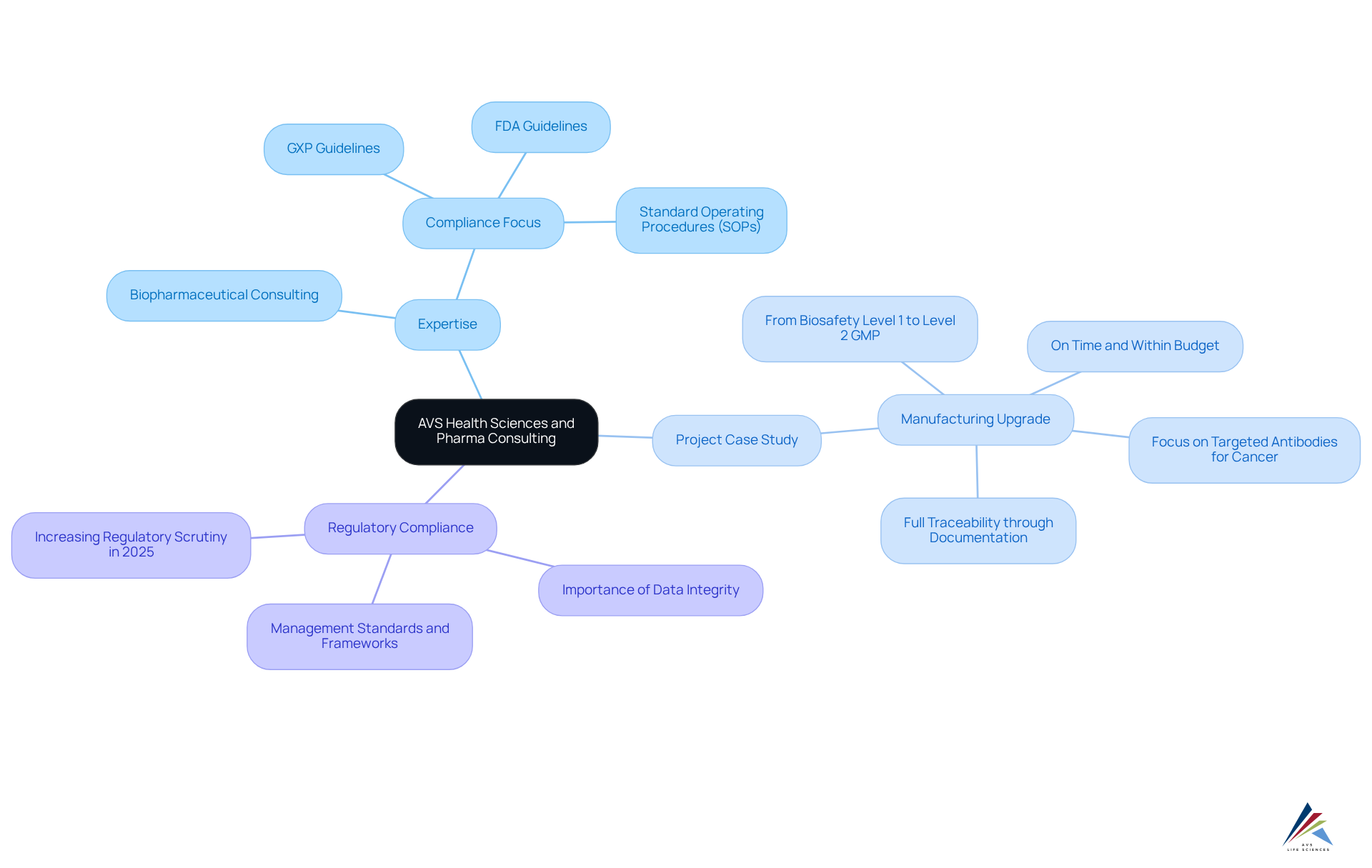
AVS Health Sciences, recognized among the top pharma consulting firms, leverages its extensive expertise in biopharmaceutical consulting to provide comprehensive solutions that significantly enhance success in the pharmaceutical sector. By merging deep industry knowledge with a strong focus on compliance—including GXP, FDA guidelines, and standard operating procedures (SOPs)—AVS empowers clients to adeptly navigate complex compliance landscapes, streamline drug development processes, and refine commercial strategies.
Notably, AVS Health Sciences played a pivotal role in assisting a leading biotechnology company in upgrading their manufacturing area from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. This project was executed on time and within budget, enabling the client to focus on the development of targeted antibodies for cancer. Throughout this collaboration, AVS ensured full traceability through meticulous documentation practices.
As the pharmaceutical sector faces increasing regulatory scrutiny in 2025, particularly concerning data integrity and compliance, insights from top pharma consulting firms like AVS Sciences regarding management standards and regulatory frameworks are vital for organizations striving for operational efficiency.
Furthermore, the emphasis on excellence in control and compliance within biotech is illustrated by AVS's transformative case study, which highlights the importance of addressing gaps in testing processes and fostering open discussions within teams to enhance overall performance.
Navigant Consulting: Strategic Advisory Services for Healthcare and Pharma
AVS Life Sciences is among the top pharma consulting firms, providing strategic consulting services meticulously tailored for the pharmaceutical and biotechnology sectors. Our team of industry specialists delivers comprehensive GXP compliance services, focusing on excellence in adherence and validation solutions throughout the drug development lifecycle.
With a strong emphasis on GMP audits—encompassing API & Drug Product CMOs, Contract Test Labs, and Data Integrity—we ensure compliance in testing facilities, guiding clients through complex regulatory landscapes.
A recent case study underscores our expertise:
- We successfully upgraded a biotechnology GMP facility, assisting a leading San Francisco-based biotechnology company in transitioning from a Biosafety Level 1 to a Level 2 GMP facility.
- This project was completed on time and within budget, exemplifying our commitment to excellence and operational efficiency.
By partnering with AVS Life Sciences, organizations can enhance their operational capabilities and compliance frameworks, which aligns with the best practices of top pharma consulting firms, ultimately improving patient wellbeing.

PAREXEL International: Regulatory Consulting and Clinical Research Expertise
AVS Healthcare delivers comprehensive compliance and assurance solutions across the biopharmaceutical, medical device, and nutraceutical sectors. Recognizing the complexities of international compliance requirements, AVS Sciences empowers clients to navigate the intricacies of adherence and assurance throughout the drug development process. Their specialization in Good Manufacturing Practices (GMP) enables clients to effectively manage risks and streamline their pathways to market, establishing AVS as a trusted partner in the life sciences sector.
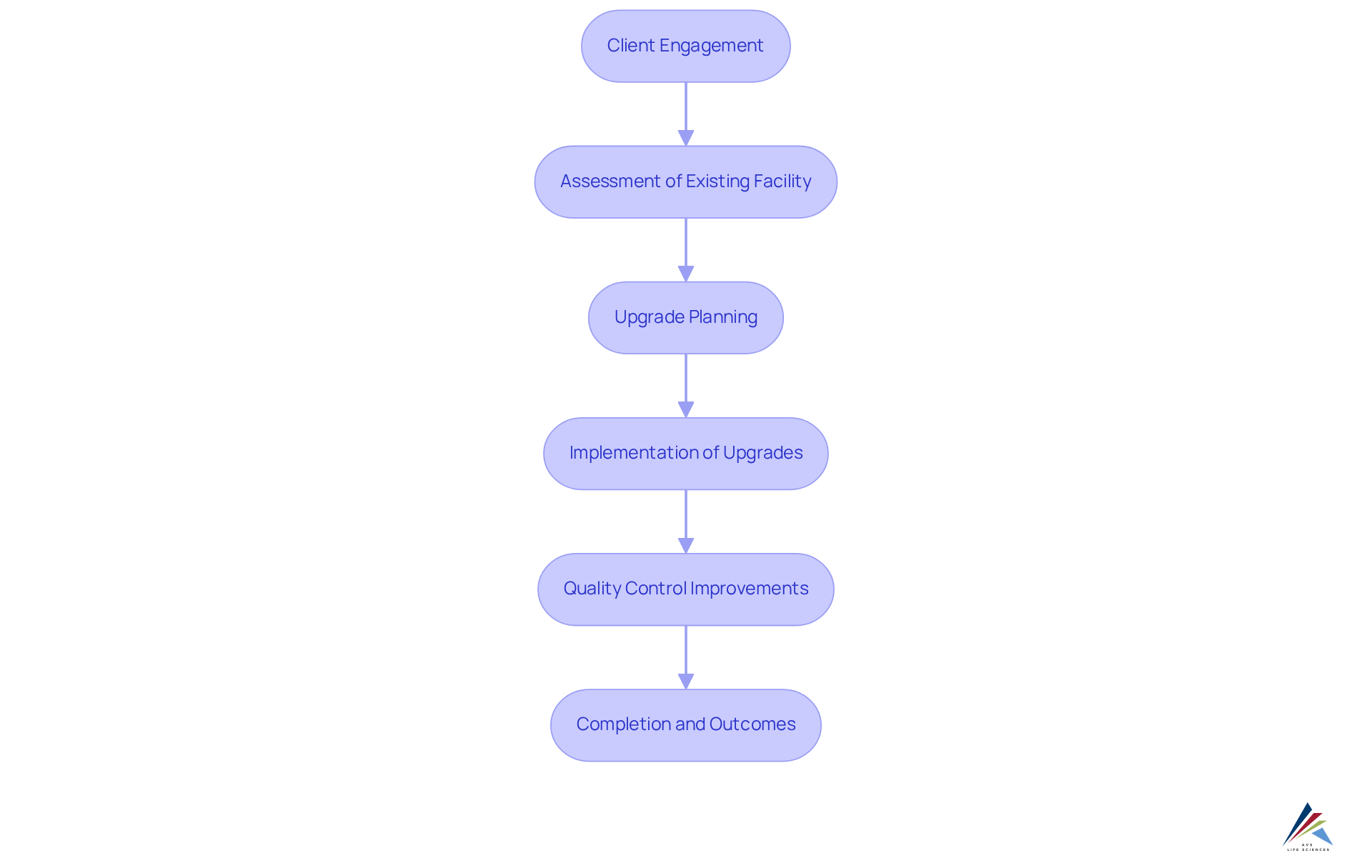
A notable case study exemplifies AVS Life Sciences' successful enhancement of a biotechnology GMP facility, where they supported a leading biotechnology company in San Francisco in upgrading from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. This project was completed on schedule and within budget, underscoring AVS's commitment to excellence and compliance standards. The collaboration allowed the client to concentrate on developing medicines that enhance patient quality of life while gleaning valuable lessons that improved their quality control processes.
As the biopharmaceutical sector faces evolving challenges in 2025, including the need for robust governance strategies, AVS Sciences' expertise is crucial for organizations striving to maintain compliance and achieve operational excellence.
Deloitte: Comprehensive Consulting Services for Life Sciences
AVS Life Sciences offers a comprehensive suite of consulting services tailored for the health sciences sector, which emphasizes compliance with regulations, operational efficiency, and strategic growth, establishing itself as one of the top pharma consulting firms in biopharmaceuticals, medical devices, and nutraceuticals.
Our team of industry experts collaborates closely with pharmaceutical and biotech companies to navigate the complex compliance landscape, streamline processes, and drive innovation.
With a steadfast commitment to excellence and adherence to regulations, AVS Life Sciences has successfully assisted clients in upgrading their GMP facilities, ensuring they align with the highest standards throughout the drug development lifecycle.
Our implementation of digitized batch processing solutions significantly boosts manufacturing efficiency, empowering clients to achieve sustainable growth and compliance in a competitive environment.
As one client remarked, 'AVS Life Sciences has been essential in our transition to a higher GMP standard, allowing us to focus on developing therapies that are vital for survival.

McKinsey & Company: Strategic Consulting for Pharma Innovation
AVS Health Sciences is considered one of the top pharma consulting firms in the biopharmaceutical sector, recognized for its extensive consulting services that encompass standards management, regulatory compliance, and engineering solutions. Our team works in close collaboration with clients to develop strategies that not only enhance operational efficiency but also drive innovation, ultimately leading to improved patient outcomes.
By leveraging data-informed decision-making alongside comprehensive market analysis, AVS Health Sciences equips pharmaceutical firms with essential tools to navigate complex compliance landscapes, including GXP and FDA guidelines, while seizing emerging opportunities.
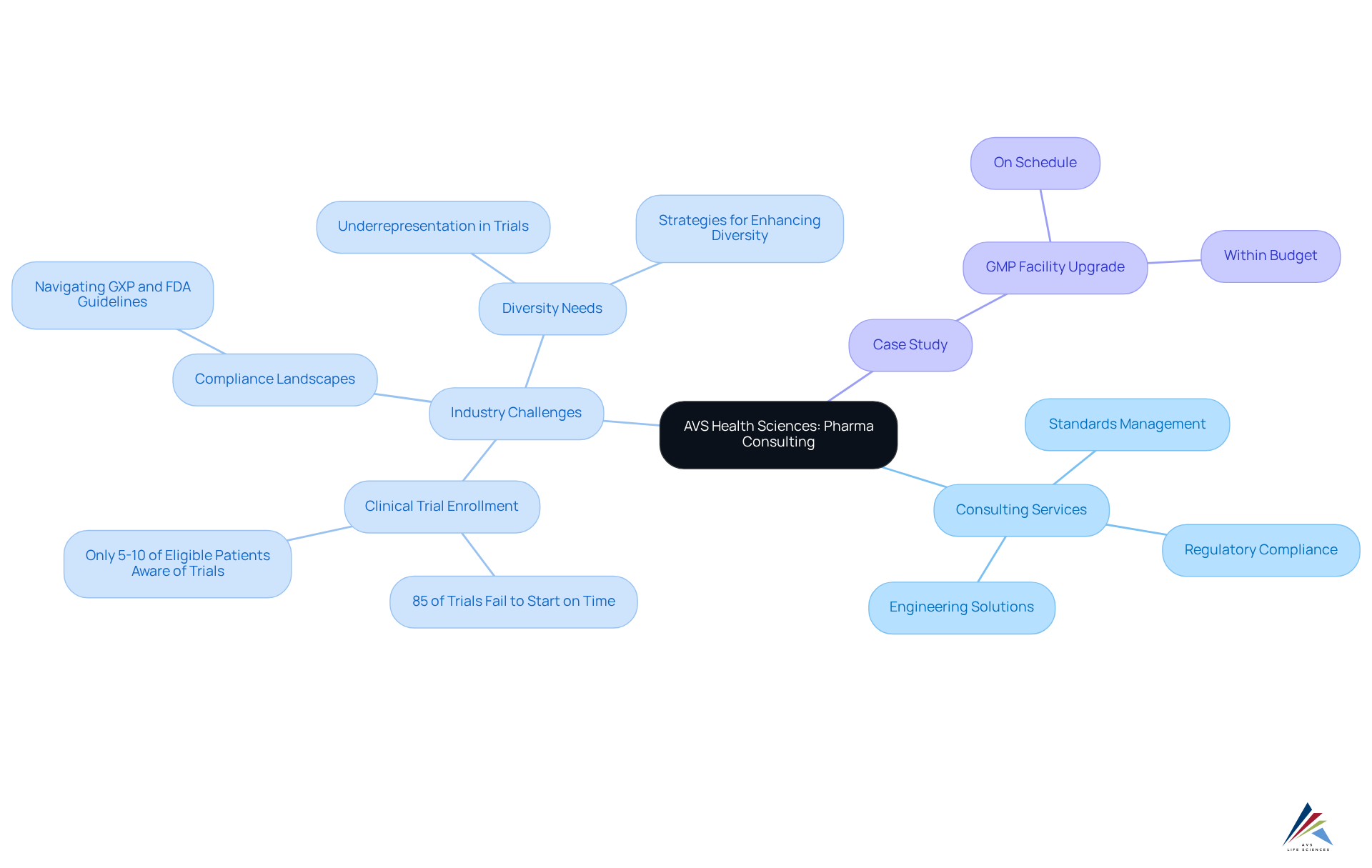
The industry faces significant challenges; notably, around 85% of clinical trials fail to commence on time due to enrollment issues, underscoring the urgent need for top pharma consulting firms to provide effective consulting services that streamline processes and enhance timelines. Our strategic approach is crucial in tackling these evolving challenges, particularly the demand for greater clinical trial diversity, which fosters better healthcare solutions and enriches patient experiences.
A transformative case study that illustrates our impact involved supporting a leading biotechnology company in upgrading their GMP facility from Biosafety Level 1 to Level 2. This project was completed on schedule and within budget, with our documentation efforts receiving commendations from the client’s assurance team. This partnership empowered our client to focus on developing treatments that significantly improve patients' quality of life, thereby showcasing our commitment to operational excellence and regulatory adherence.
ZS Associates: Sales and Marketing Consulting for Pharma Optimization
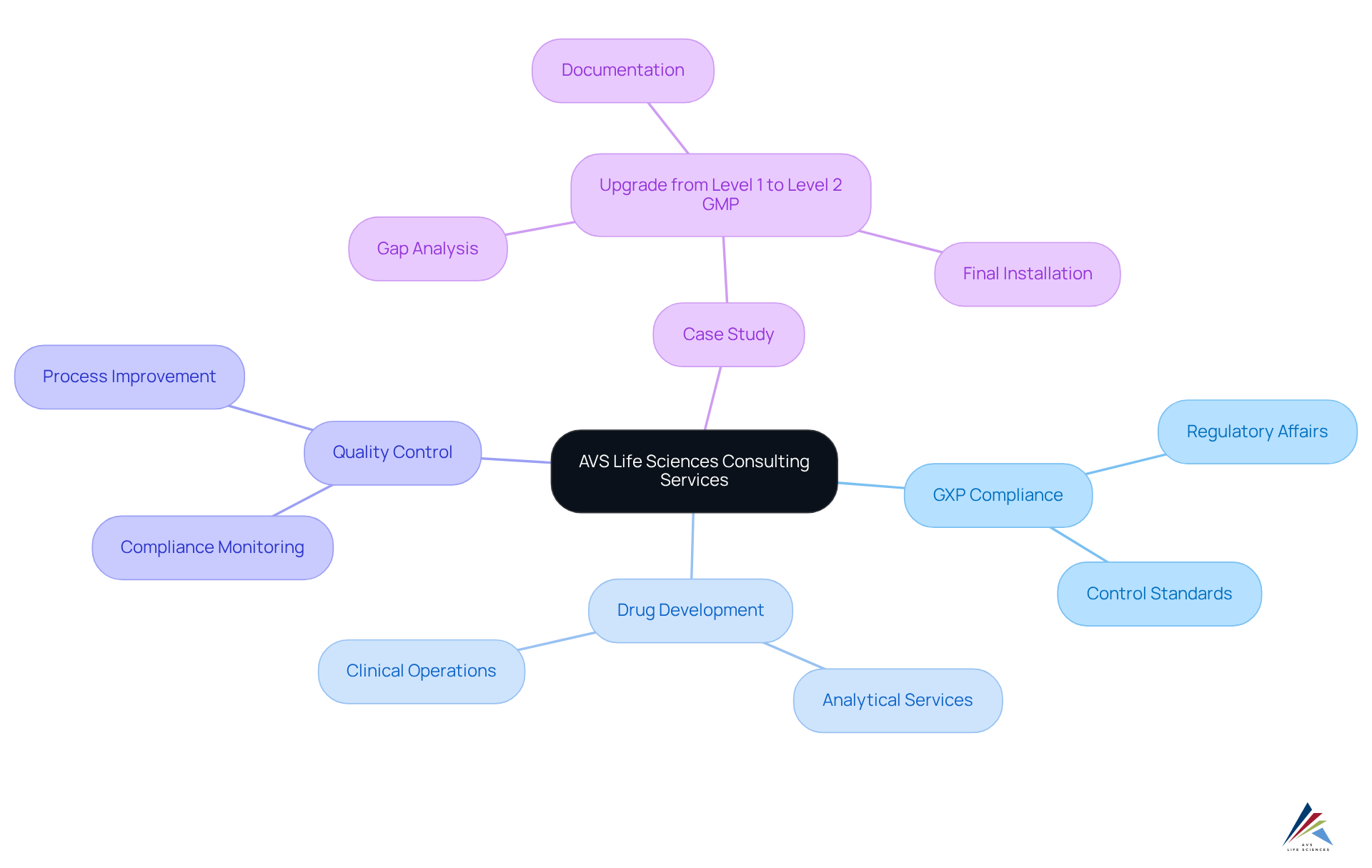
AVS Life Sciences is recognized as one of the top pharma consulting firms, specializing in comprehensive GXP compliance services and assurance solutions tailored for the pharmaceutical and biotechnology sectors. Our extensive biopharma offerings, provided by top pharma consulting firms, span every phase of drug development, ensuring excellence and regulatory adherence throughout the product lifecycle. We deliver customized solutions that meet our clients' unique requirements, which are often developed in collaboration with top pharma consulting firms, encompassing analytical services, control standards, CMC, regulatory affairs, and clinical operations.
A transformative case study that underscores our expertise involved aiding a distinguished biotechnology company based in San Francisco in the upgrade of their manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. This project was completed on schedule and within budget, with our meticulous documentation efforts providing complete traceability, which was highly valued by the client’s assurance team. Our extensive support throughout the transition—from gap analysis to final equipment installation—empowered the client to concentrate on developing innovative treatments for serious diseases.
Furthermore, our unwavering commitment to enhancing quality control and compliance was evident when we identified discrepancies in test results during the upgrade. This discovery initiated critical discussions regarding business processes and team responsibilities, ultimately bolstering their operational integrity. AVS Life Sciences is resolute in ensuring that your systems, processes, and facilities remain compliant with the highest industry standards, enabling you to deliver safe and effective therapies to patients.
Accenture: Technology and Consulting Solutions for Pharma Innovation
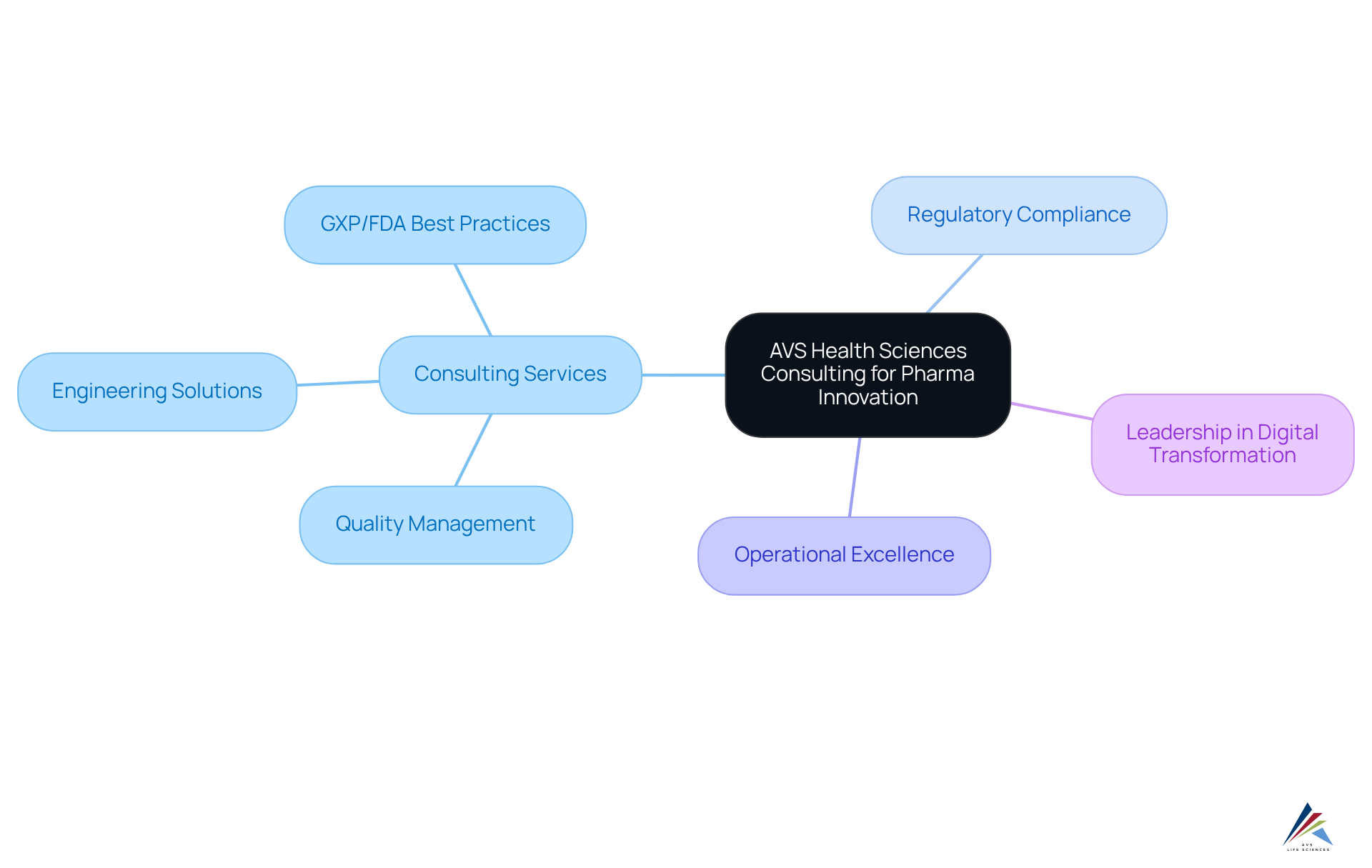
AVS Health Sciences offers a comprehensive range of consulting services designed to foster innovation within top pharma consulting firms. Their profound expertise in quality management, regulatory compliance, and engineering solutions empowers clients to streamline operations and enhance patient outcomes.
As the biopharmaceutical market evolves rapidly, AVS Health Sciences prioritizes leveraging best practices in GXP, FDA regulations, and standard operating procedures (SOPs) to position pharmaceutical companies for sustainable growth, collaborating with top pharma consulting firms.
A notable example of their impact is the successful upgrade of a biotechnology GMP facility, which exemplifies the transformative potential of their consulting services. This project not only ensured compliance with stringent guidelines but also advanced operational excellence, allowing clients to focus on developing life-saving medicines.
Furthermore, AVS Health Sciences emphasizes that leadership must actively engage in digital transformation and excellence management to improve operational efficiency and foster patient involvement.
Bain & Company: Performance Enhancement Consulting for Pharma
AVS Sciences excels in delivering comprehensive GXP compliance services tailored specifically for top pharma consulting firms in the pharmaceutical and biotechnology sectors. By focusing on quality adherence and validation solutions throughout the drug development lifecycle, they address the pressing compliance challenges faced by industry players. Their expert team collaborates closely with pharmaceutical organizations to identify improvement opportunities, craft actionable strategies, and implement solutions that enhance operational efficiency and regulatory compliance. Leveraging data-driven insights and adhering to industry best practices, AVS Sciences positions itself as a reliable partner for companies striving to elevate their performance in an increasingly competitive landscape.
Key Highlights:
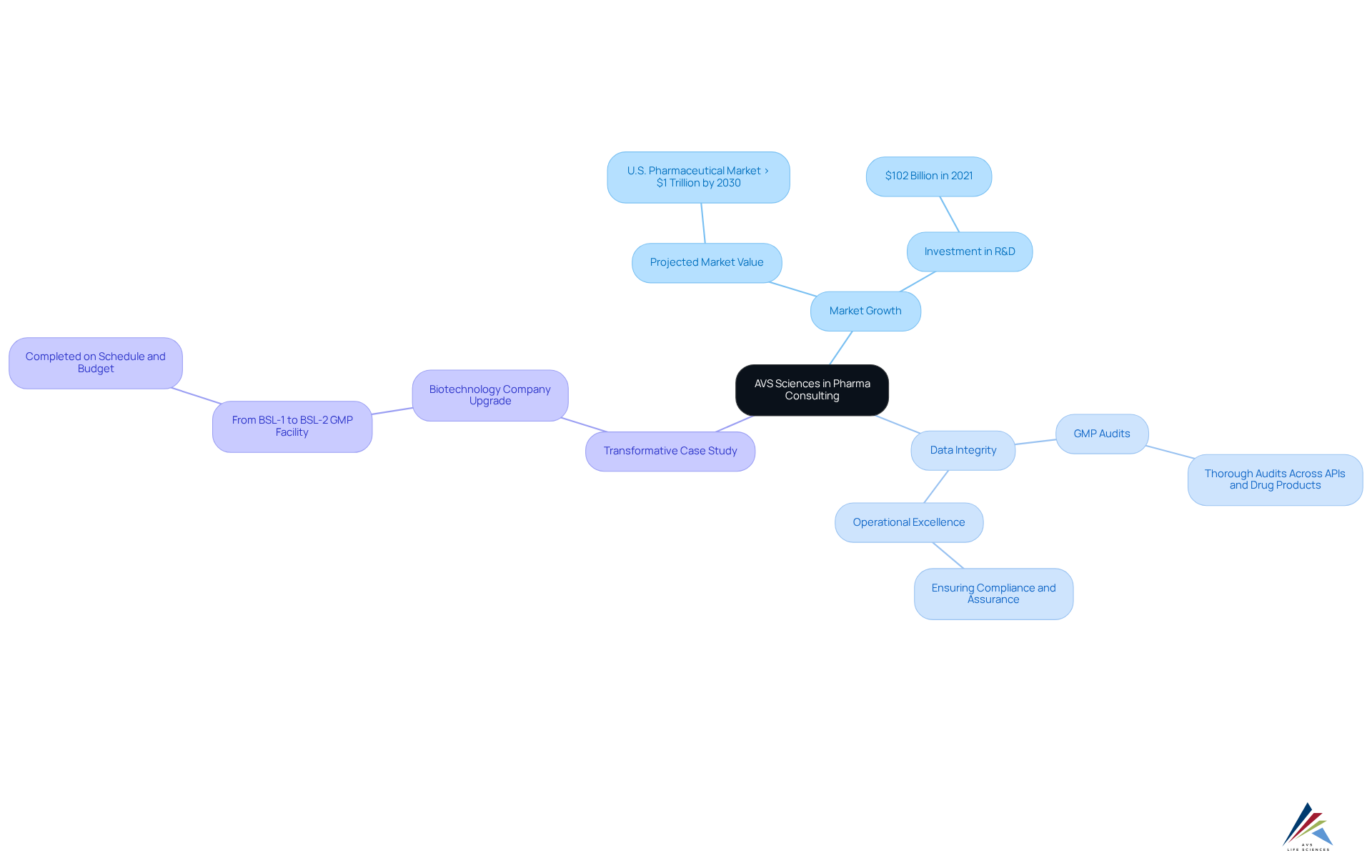
- Market Growth: The U.S. pharmaceutical market is projected to exceed $1 trillion by 2030, emphasizing the need for operational improvements to capitalize on this growth.
- Data Integrity: AVS Sciences' dedication to operational excellence is highlighted by their thorough GMP audits across APIs, drug products, and testing facilities, ensuring compliance and assurance.
- Transformative Case Study: A notable success story involves AVS assisting a leading biotechnology company in upgrading their manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. This project was completed on schedule and within budget, demonstrating AVS's capability in supporting clients through critical transitions while maintaining compliance and operational excellence.
By integrating these elements, AVS Sciences not only enhances its consulting effectiveness but also collaborates with top pharma consulting firms to support pharmaceutical firms in navigating the complexities of compliance and operational efficiency.
Charles River Associates: Economic and Financial Consulting for Pharma Challenges
AVS Health Sciences stands out as a leading provider of comprehensive management and compliance solutions tailored for the pharmaceutical industry. The team of specialists adeptly navigates the complex landscape of legal requirements, ensuring that pharmaceutical companies can effectively tackle compliance challenges. By harnessing data-driven decision-making and advanced quality management practices, AVS Sciences empowers these companies to make informed choices that promote sustainable growth in an increasingly competitive environment.
As the industry evolves, AVS Sciences' unwavering commitment to delivering actionable insights enables clients to adeptly address the challenges posed by regulatory dynamics, ultimately enhancing their strategic positioning and operational success.
Furthermore, AVS Life Sciences is resolutely dedicated to fostering diversity, equity, and inclusion within the life sciences consulting realm, acknowledging the critical role of diverse perspectives in driving innovation and achieving compliance excellence.

Conclusion
AVS Life Sciences and other leading consulting firms within the pharmaceutical sector play a pivotal role in ensuring compliance excellence and operational efficiency. These firms are not only skilled at navigating the complex regulatory landscape but also empower their clients to innovate and excel in the competitive biopharmaceutical market. By leveraging their extensive expertise, they assist organizations in streamlining processes, enhancing quality management, and ultimately improving patient outcomes.
This article has presented key insights on various consulting firms, including AVS Life Sciences, IQVIA, Navigant Consulting, and others. Each firm offers unique strengths; for instance:
- AVS provides comprehensive regulatory solutions
- IQVIA delivers data-driven insights
- Deloitte emphasizes operational efficiency
Noteworthy case studies underscore successful projects that exemplify how these firms enable clients to transition to higher compliance standards, fostering a culture of excellence within the industry.
As the pharmaceutical landscape continues to evolve, the importance of partnering with experienced consulting firms cannot be overstated. Organizations must prioritize compliance and operational excellence to thrive in an environment characterized by increasing regulatory scrutiny and market demands. Engaging with top pharma consulting firms not only enhances a company's ability to meet compliance standards but also positions them for sustainable growth and innovation in the years to come.
Frequently Asked Questions
What services does AVS Life Sciences provide?
AVS Life Sciences offers management solutions, regulatory compliance, engineering, automation, validation, and clinical services specifically for the pharmaceutical, biotechnology, medical device, and diagnostic sectors.
How does AVS Life Sciences assist clients in the life sciences sector?
AVS Life Sciences empowers clients throughout the product lifecycle by helping them navigate complex compliance landscapes while maintaining rigorous standards.
What are the core services offered by AVS Life Sciences?
The core services include validation and commissioning, compliance consulting, engineering assistance, and submissions guidance.
What is the size and expertise of the AVS Life Sciences team?
AVS Life Sciences has a dedicated team of over 300 skilled professionals worldwide.
What recent acquisition has enhanced AVS Life Sciences' capabilities?
The recent acquisition of ValidPath has enhanced AVS's engineering capabilities.
What is the significance of AVS Life Sciences' 80% repeat business rate?
The 80% repeat business rate indicates a strong client satisfaction and trust in AVS Life Sciences as a reliable partner in the life sciences domain.
What trends and compliance expectations is AVS Life Sciences preparing for in 2025?
AVS Life Sciences is implementing robust quality management strategies that align with the evolving trends and compliance expectations in the pharmaceutical landscape for 2025.
How does AVS Health Sciences support clients in the pharmaceutical sector?
AVS Health Sciences provides comprehensive solutions that enhance success by focusing on compliance with GXP, FDA guidelines, and streamlining drug development processes.
Can you provide an example of a project handled by AVS Health Sciences?
AVS Health Sciences assisted a leading biotechnology company in upgrading their manufacturing area from a Biosafety Level 1 GMP facility to a Level 2 GMP facility, completing the project on time and within budget.
Why is compliance and data integrity important in the pharmaceutical sector?
Compliance and data integrity are crucial due to increasing regulatory scrutiny, especially concerning operational efficiency and adherence to management standards in the pharmaceutical sector.
What role does AVS Life Sciences play in enhancing operational capabilities for organizations?
By partnering with AVS Life Sciences, organizations can improve their operational capabilities and compliance frameworks, aligning with best practices in the industry and ultimately enhancing patient wellbeing.
