10 Essential Insights on ICH M10 for Compliance Officers

Overview
This article provides essential insights into the ICH M10 guidelines, specifically tailored for compliance officers. It underscores the critical role these guidelines play in ensuring rigorous bioanalytical method validation. By adhering to these standards, organizations can significantly enhance data integrity and regulatory compliance. Evidence from within the life sciences sector demonstrates that such adherence not only improves practices but also fosters collaboration among stakeholders, ultimately driving better outcomes. Engaging with these guidelines is not merely a regulatory requirement; it is a strategic imperative for maintaining excellence in bioanalytical methodologies.
Introduction
The introduction of ICH M10 guidelines signifies a pivotal moment in bioanalytical method validation, effectively addressing long-standing inconsistencies in global practices. This article explores ten essential insights that compliance officers can utilize to deepen their understanding and implementation of these guidelines, ultimately enhancing data integrity and regulatory compliance. As organizations endeavor to align with these stringent standards, they encounter distinct challenges. What key obstacles must compliance officers navigate to ensure successful adherence to ICH M10?
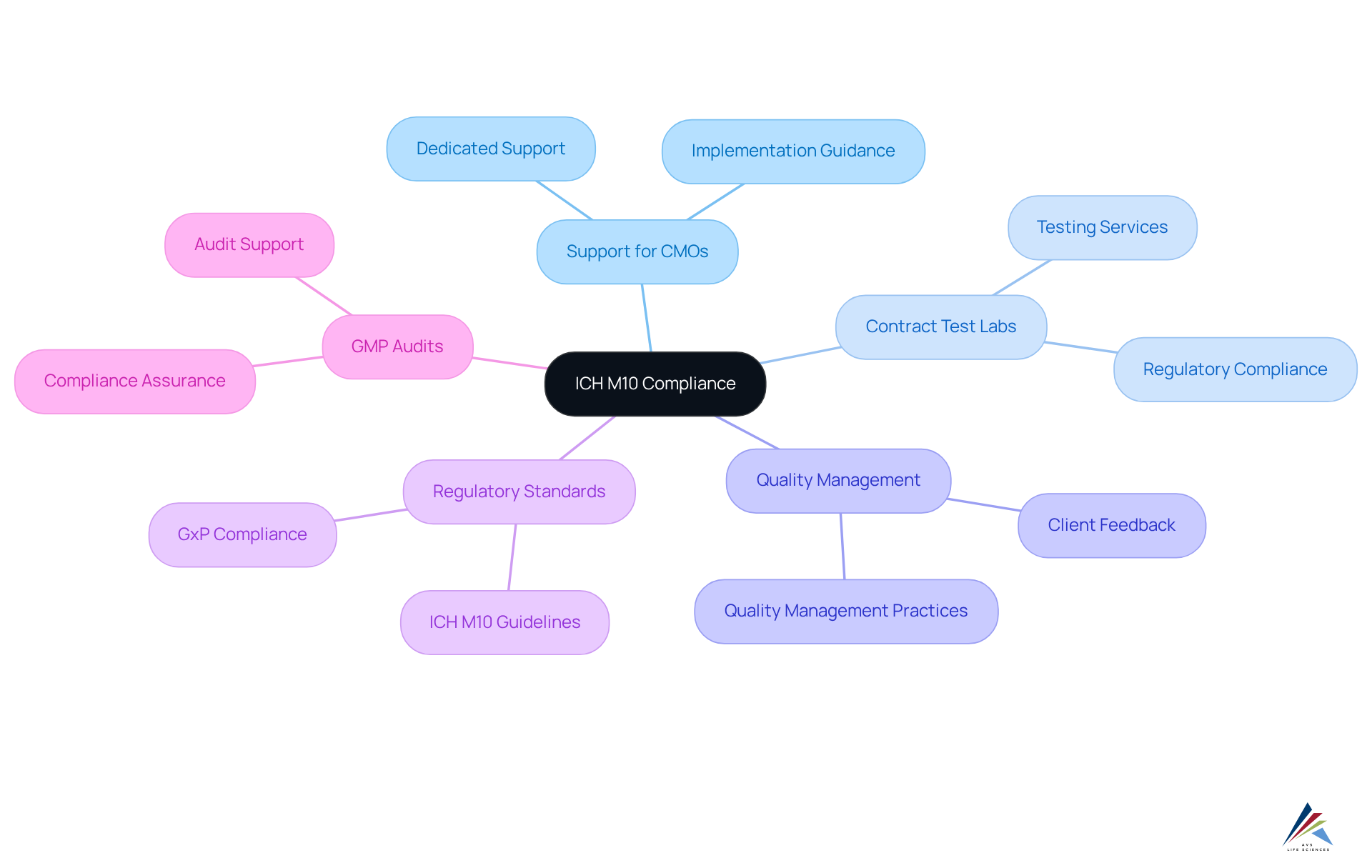
AVS Life Sciences: Comprehensive Solutions for ICH M10 Compliance
AVS Life Sciences presents a robust suite of services designed to ensure strict adherence to ICH M10 standards. This includes dedicated support for API & Drug Product CMOs and Contract Test Labs. Leveraging their extensive knowledge in quality management and regulatory standards, clients can seamlessly implement the guidelines established by ICH M10. Such guidance is essential for enhancing quality management practices within the life sciences sector, enabling organizations to adeptly navigate the complexities of bioanalytical method assessment.
With a pronounced emphasis on GMP audits, AVS Life Sciences assures compliance across APIs, drug products, and testing facilities. This positions them as a premier provider of consulting and verification services for GxP, ISO, and QSR within the life sciences domain. Their proven track record, underscored by positive client feedback and a steadfast commitment to quality management, empowers compliance officers to uphold the highest standards throughout the assessment process.
Historical Development of ICH M10 Guidelines
The ich m10 guidelines were established to address discrepancies in bioanalytical method assessment across various regions, a necessity recognized by stakeholders since 2010. This collaborative initiative culminated in the adoption of the finalized guidelines in May 2022, marking a pivotal advancement in harmonizing global practices in bioanalytical method validation.
Regulatory specialists assert that embracing ich m10 is crucial for ensuring compliance and fostering trust in bioanalytical data, which is essential for drug development and regulatory submissions. Notably, statistics reveal that approximately 58% of organizations have initiated the implementation of these guidelines, indicating a significant shift towards standardized practices.
Understanding this historical context is vital for regulatory officers, as it underscores the evolution of regulatory expectations and highlights the importance of adhering to these guidelines for successful product development and market approval.
At AVS Life Sciences, we leverage our extensive industry expertise to provide tailored verification, quality assurance, and regulatory solutions, empowering officers to navigate these guidelines efficiently and ensuring that organizations meet the stringent standards required in the life sciences sector.

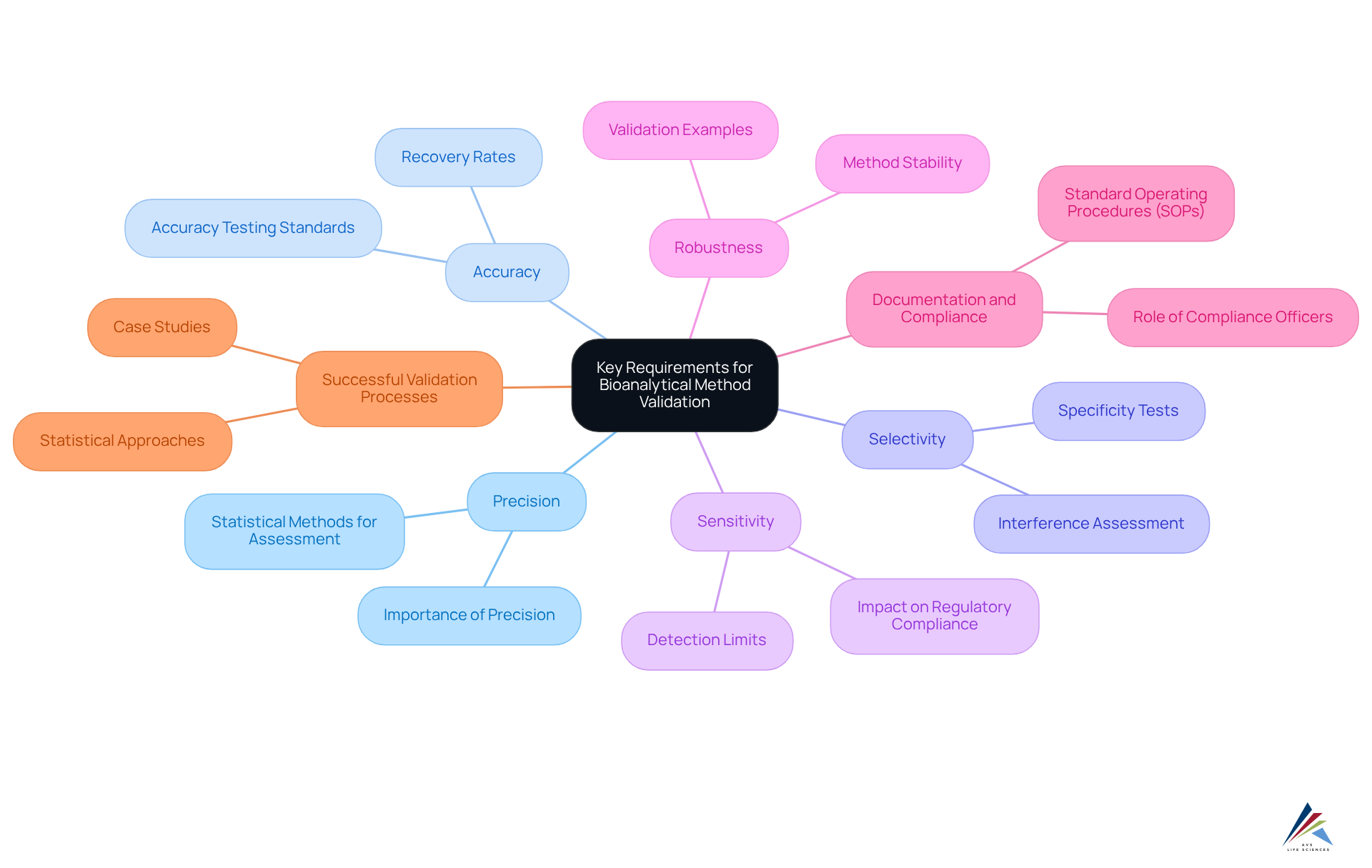
Key Requirements for Bioanalytical Method Validation Under ICH M10
The essential requirements for bioanalytical method assessment, including precision, accuracy, selectivity, sensitivity, and robustness, are established by ICH M10. Compliance officers play a pivotal role in ensuring that these methods are rigorously validated to produce reliable data for regulatory submissions. The guidelines emphasize the importance of detailed documentation methods and the creation of Standard Operating Procedures (SOPs) for all verification processes and outcomes, which are crucial for ensuring adherence and supporting audits.
AVS Life Sciences provides expert solutions in GMP adherence, verification, and engineering, ensuring that organizations follow FDA regulations and GXP standards. Recent statistics suggest that adherence to ICH M10 verification parameters significantly enhances compliance rates. Studies demonstrate that methods meeting these criteria exhibit a failure probability of less than 1% when true total coefficients of variation (CV) are maintained at 10%. Furthermore, successful confirmation processes, such as those involving high-performance liquid chromatography (HPLC) for levofloxacin, illustrate that assessment parameters can be achieved within acceptance limits, yielding an assay of 99.06% and a % RSD value of 1.28%. These examples underscore the effectiveness of adhering to ICH M10 guidelines in achieving robust and reliable bioanalytical methods, ultimately supporting regulatory standards and product quality.
Moreover, understanding the definition of a comparative bioavailability study under ICH M10 is crucial for regulatory officers who manage the intricacies of verification. The recent 'Hot Topic' session held in October 2023 discussed the challenges in implementing ICH M10, emphasizing the necessity of early communication with regulatory agencies during drug development. This ongoing dialogue is essential for ensuring that adherence measures align with regulatory expectations, reinforcing AVS Life Sciences' commitment to providing comprehensive quality management and regulatory adherence solutions for the life sciences sector.
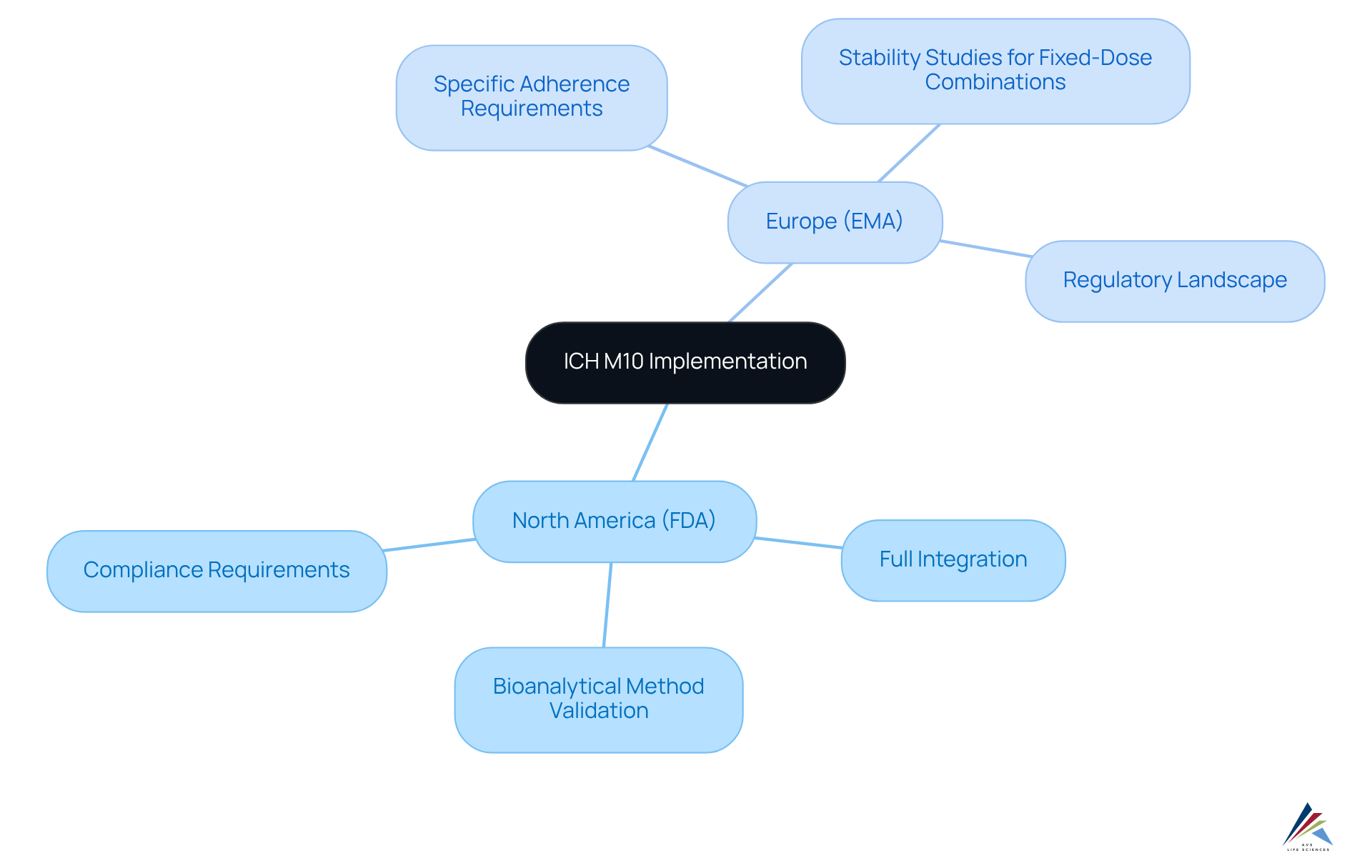
Global Implementation of ICH M10: Regional Perspectives
The implementation of ich m10 guidelines has exhibited notable regional variation, particularly as the FDA and EMA embraced these standards in 2023. In North America, the FDA has fully integrated ich m10 into its regulatory framework, highlighting the necessity for robust bioanalytical method validation. The official acceptance of the ich m10 guideline in May 2022 marked a pivotal moment for adherence in the pharmaceutical sector. Conversely, the EMA has delineated specific adherence requirements that reflect the distinct regulatory landscape in Europe, including the imperative for stability studies for fixed-dose combinations.
Compliance officers must remain vigilant regarding these regional differences, as they are vital for ensuring that their organizations meet the necessary standards for bioanalytical method assessment across diverse markets. This awareness not only facilitates compliance with regulatory expectations but also enhances the overall quality and reliability of bioanalytical data submitted for drug approval.
Moreover, the public consultation on the implementation strategy that concluded on January 31, 2024, highlights the industry's proactive engagement with these guidelines. However, the challenges stemming from the absence of specified acceptance criteria for cross-validation present potential hurdles in implementation according to ich m10. Understanding the influence of bioanalytical data on regulatory decisions is essential for officers to appreciate the broader implications of adhering to the ich m10 guidelines.
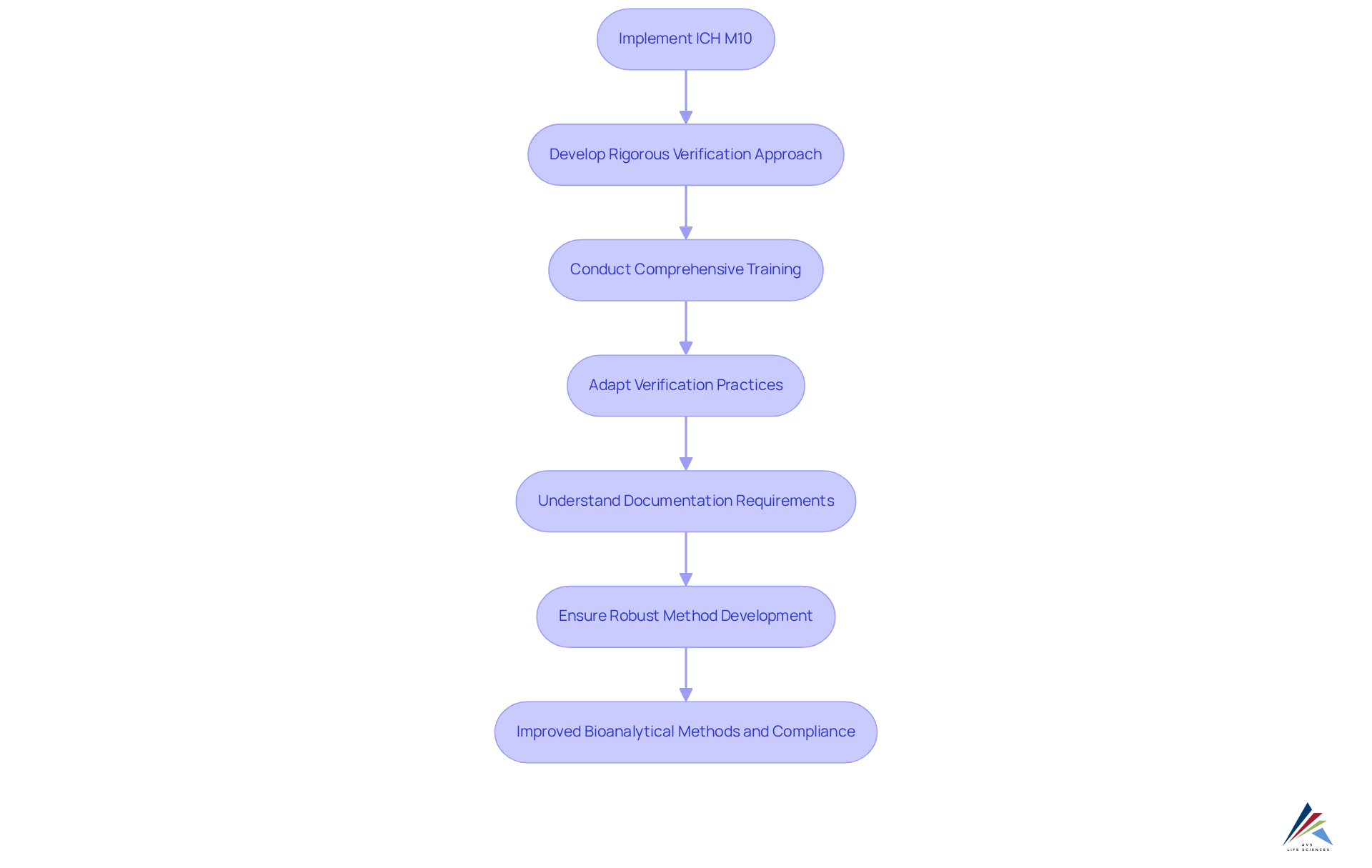
Impact of ICH M10 on Method Development and Validation Practices
The implementation of ICH M10 has significantly transformed method development and assessment practices within the pharmaceutical sector. Organizations are now required to implement a more rigorous verification approach, ensuring that methods not only meet regulatory standards but also yield dependable and consistent results. This evolution necessitates comprehensive training programs tailored to equip staff with the necessary skills and knowledge for compliance with ICH M10 guidelines. Enhanced training initiatives may include workshops focused on statistical methods for cross-validation, as well as practical sessions on the preparation of calibration standards and quality control samples.
Industry leaders have observed that the shift towards ICH M10 has resulted in significant changes in verification practices. A notable quotation from a prominent authority highlights, "The ICH M10 guidance is a positive advancement towards a standardized global framework of requirements for bioanalytical method development, method assessment, sample analysis, and reporting." This sentiment reflects a broader consensus on the importance of aligning validation practices with international standards.
Furthermore, training requirements for adherence to ICH M10 now include a thorough understanding of the new documentation practices mandated by the ICH M10 guidance. This includes detailed records of sample reintegration processes and the rationale behind any deviations from established protocols. As organizations adapt to these changes, the focus on robust bioanalytical method development becomes paramount, ensuring that all methods are validated to meet the rigorous expectations set forth by regulatory agencies. The emphasis on continuous training and adaptation will ultimately enhance the reliability of bioanalytical results, facilitating smoother regulatory approvals and fostering innovation in drug development.
Incurred Sample Reanalysis (ISR) Requirements in ICH M10
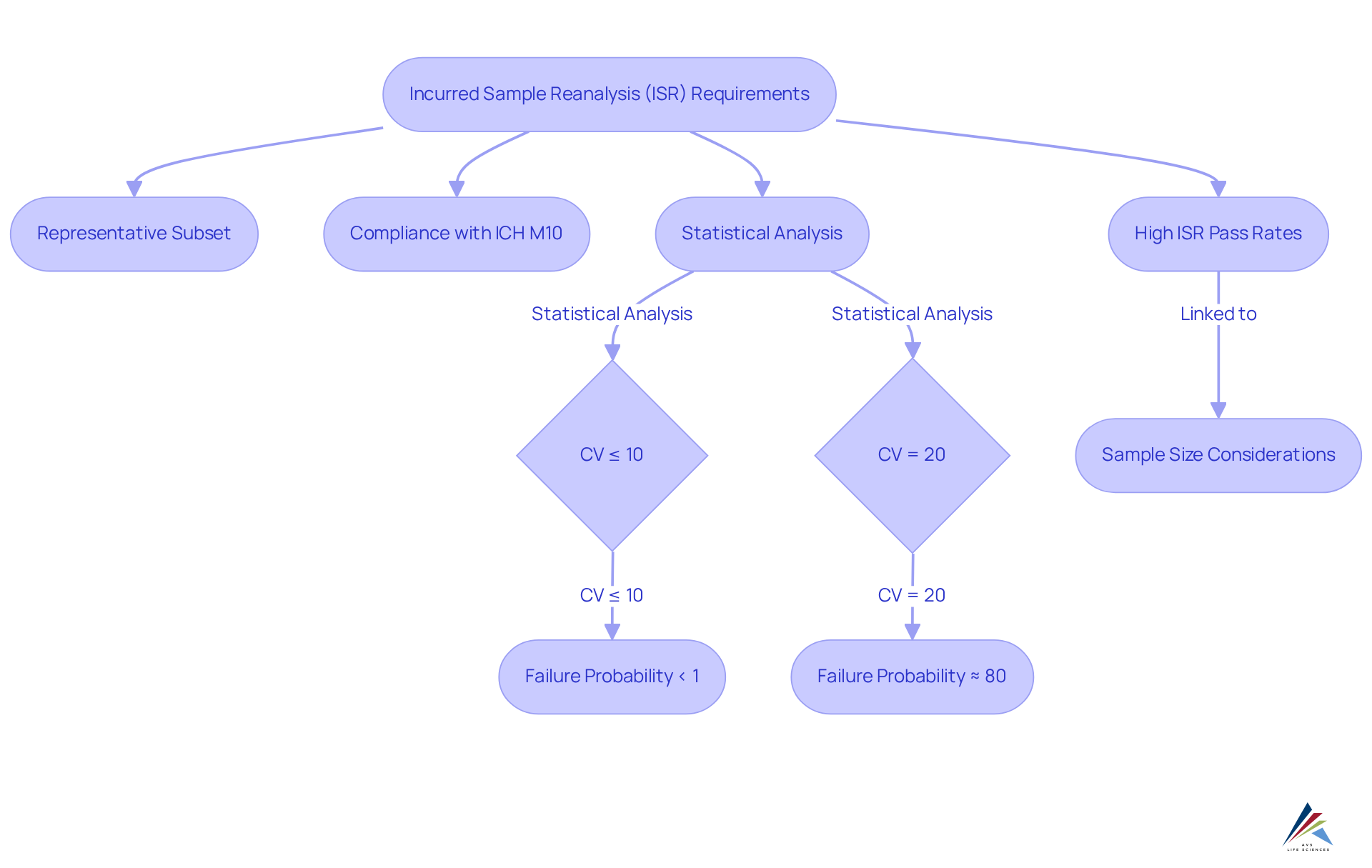
Incurred Sample Reanalysis (ISR) is a fundamental requirement outlined in ICH M10, which is designed to verify the reliability of reported analyte concentrations. Compliance officers are tasked with ensuring that ISR is conducted on a representative subset of incurred samples, confirming that results are both consistent and reproducible. This process is vital for maintaining data integrity, as it directly impacts the credibility of bioanalytical methods in meeting regulatory expectations.
Expert opinions underscore the significance of ISR in alignment with ICH M10, emphasizing that rigorous adherence to ISR protocols not only enhances the reliability of bioanalytical data but also mitigates risks associated with ICH M10 regulatory scrutiny. Statistical analyses indicate that techniques with a real total coefficient of variation (CV) of 10% or lower yield a failure probability of under 1% when subjected to ISR, thus highlighting the necessity of rigorous verification practices. Conversely, for methods exhibiting a true total CV of 20%, the probability of ISR test failure can soar to as high as 80% with a sample size of 20 incurred samples, reinforcing the critical need to maintain low CVs in bioanalytical methods.
Successful implementations of ISR in bioanalytical studies have demonstrated its effectiveness in ensuring data integrity. For instance, a comprehensive evaluation of 37 studies utilizing ligand-binding assays (LBAs) revealed that all ISR studies met current regulatory criteria, with 95% passing modified Bland-Altman (mBA) criteria. This underscores the essential role of ISR in confirming the reproducibility of bioanalytical results, ultimately supporting robust regulatory submissions and fostering confidence in the integrity of the data generated. Furthermore, compliance with the ICH M10 guidelines necessitates that at least two-thirds of reanalyzed samples must fall within 30% of the original results. The significance of sample size in achieving high ISR pass rates cannot be overstated, as larger sample sizes are often imperative to sustain high pass rates and mitigate the risks of incorrectly rejecting reproducible methods due to multiple ISR testing requirements.
Dilutional Linearity Expectations in ICH M10
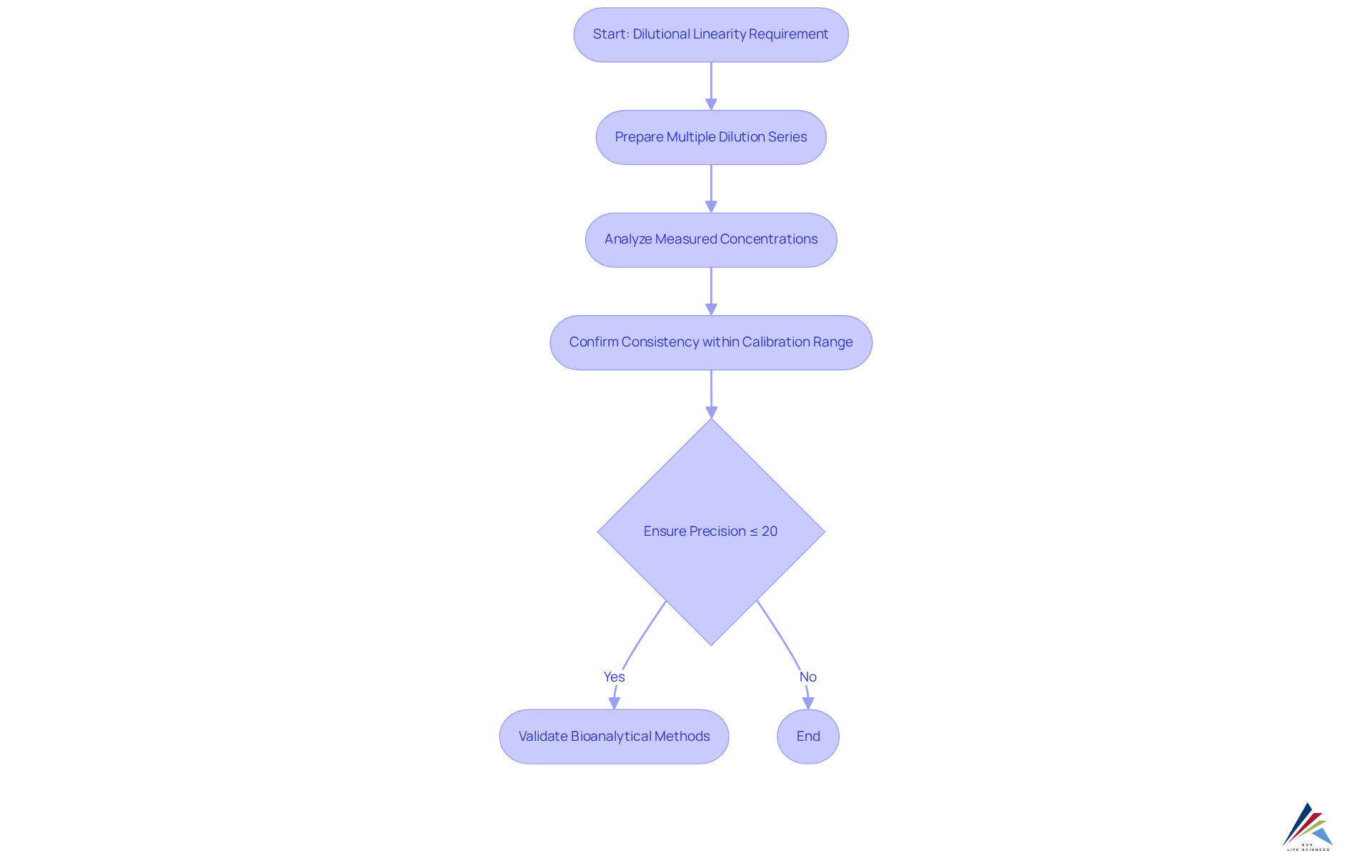
ICH M10 specifies that dilutional linearity must be demonstrated to ensure accurate measurement of high concentrations of analytes after dilution. Compliance officers are tasked with implementing studies that assess dilutional linearity by preparing multiple dilution series and analyzing them to confirm that measured concentrations remain consistent within the calibration range. Precision in dilutional linearity studies must not exceed 20%, a critical threshold for ensuring accurate quantification of samples. This requirement is essential for validating bioanalytical methods and ensuring compliance with regulatory standards.
The successful confirmation of high concentration analytes has been demonstrated in various studies, underscoring the importance of maintaining dilutional linearity. For instance, a study on dilutional linearity highlighted that achieving reliable results necessitates careful assessment of sample handling and storage conditions, as these factors can significantly influence measurement outcomes.
Industry experts assert that validating dilutional linearity transcends being a mere regulatory checkbox; it is a fundamental aspect of ensuring data integrity. Eloi P. Kpamegan, a renowned biostatistics manager, notes, "departure from dilutional similarity can indicate that the groups of organisms are not comparable or that the preparations do not contain the same active compound." This statement emphasizes the necessity for thorough verification practices. Furthermore, confirmation of biomarker assays is crucial for obtaining reliable results in clinical routines and trials.
Moreover, the ICH M10 guidelines indicate that various analytical technologies may possess differing requirements for verification parameters, complicating the establishment of a consensus protocol. Consequently, regulatory officers must ensure that their assessment strategies are robust and tailored to the unique characteristics of the analytes under examination, including the need for quality control samples that represent potential dilution factors during analysis.
In summary, demonstrating dilutional linearity is a pivotal requirement for adherence to ICH M10, which ensures that bioanalytical methods yield reliable and interpretable results in clinical settings. Compliance officers are encouraged to regularly review and refine their assessment strategies in accordance with ICH M10.
Selectivity and Stability Testing Standards in ICH M10
The critical significance of selectivity and stability testing in bioanalytical method verification is underscored by ICH M10, which is essential for ensuring compliance with GXP and FDA regulations. Compliance officers must guarantee that analytical methods can effectively distinguish analytes from potential interfering substances found in biological matrices, a crucial factor for accurate drug quantification. The guidelines dictate that selectivity assessments must encompass:
- Six sources of biological matrices for chromatographic methods
- Ten sources for ligand-binding assays
This ensures robust differentiation of analytes.
Equally vital is stability testing, as it verifies that analytes retain their integrity under various storage conditions, aligning with best documentation practices and Standard Operating Procedures (SOPs). ICH M10 delineates specific stability testing requirements, stating that:
- Small molecules require only -20°C stability testing
- Large molecules must undergo tests at both -20°C and -70°C
Adherence rates for stability testing under ICH M10 have shown improvement, reflecting the industry's commitment to these rigorous standards, with AVS Life Sciences playing a pivotal role in supporting GMP compliance and validation.
The focus on selectivity and stability not only bolsters the reliability of bioanalytical results but also aligns with regulatory expectations, ultimately facilitating successful drug development and approval processes. By implementing these standards, oversight officers can significantly enhance the integrity and quality of bioanalytical studies, reinforcing AVS Life Sciences' position as a premier provider of quality management and regulatory compliance solutions for the life sciences sector. Moreover, addressing Data Integrity Deviations, Investigations, and CAPA is crucial for upholding regulatory standards and ensuring the robustness of bioanalytical methods.

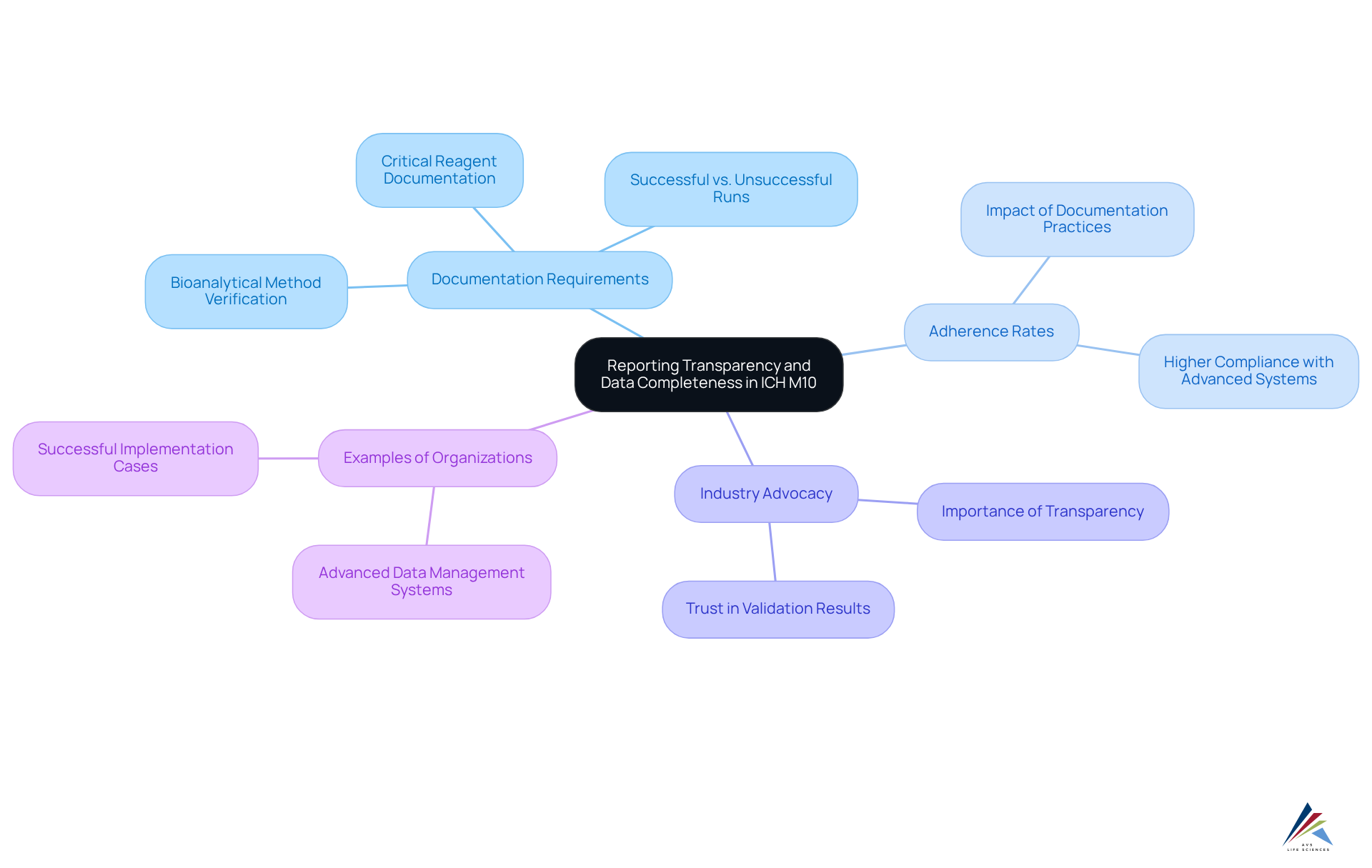
Reporting Transparency and Data Completeness in ICH M10
ICH M10 establishes stringent criteria for reporting transparency and data thoroughness, mandating that oversight officers meticulously document all information generated during bioanalytical method verification. This requirement encompasses comprehensive reporting of both accepted and unsuccessful analytical runs, which is essential for facilitating regulatory reviews and preserving the integrity of the assessment process. The guideline's emphasis on thorough documentation aims to bolster the reliability of data presented to regulatory bodies.
As we look towards 2025, organizations increasingly recognize the significance of data completeness in bioanalytical verification, with adherence rates under ICH M10 becoming a central focus for quality assurance. Industry leaders advocate for robust documentation practices, underscoring that transparency not only aids in regulatory compliance but also fosters trust in validation results. Organizations that have implemented strict documentation procedures report higher adherence rates and more seamless interactions with regulatory bodies.
Prominent examples of organizations excelling in thorough documentation for ICH M10 adherence include those employing advanced data management systems, which streamline the documentation process and ensure that all experimental data is both retrievable and verifiable in accordance with ICH M10 guidelines. Such systems not only facilitate compliance with the guideline's expectations but also contribute to more efficient regulatory submissions and enhanced data integrity.
Overall Implications of ICH M10 for Bioanalytical Scientists
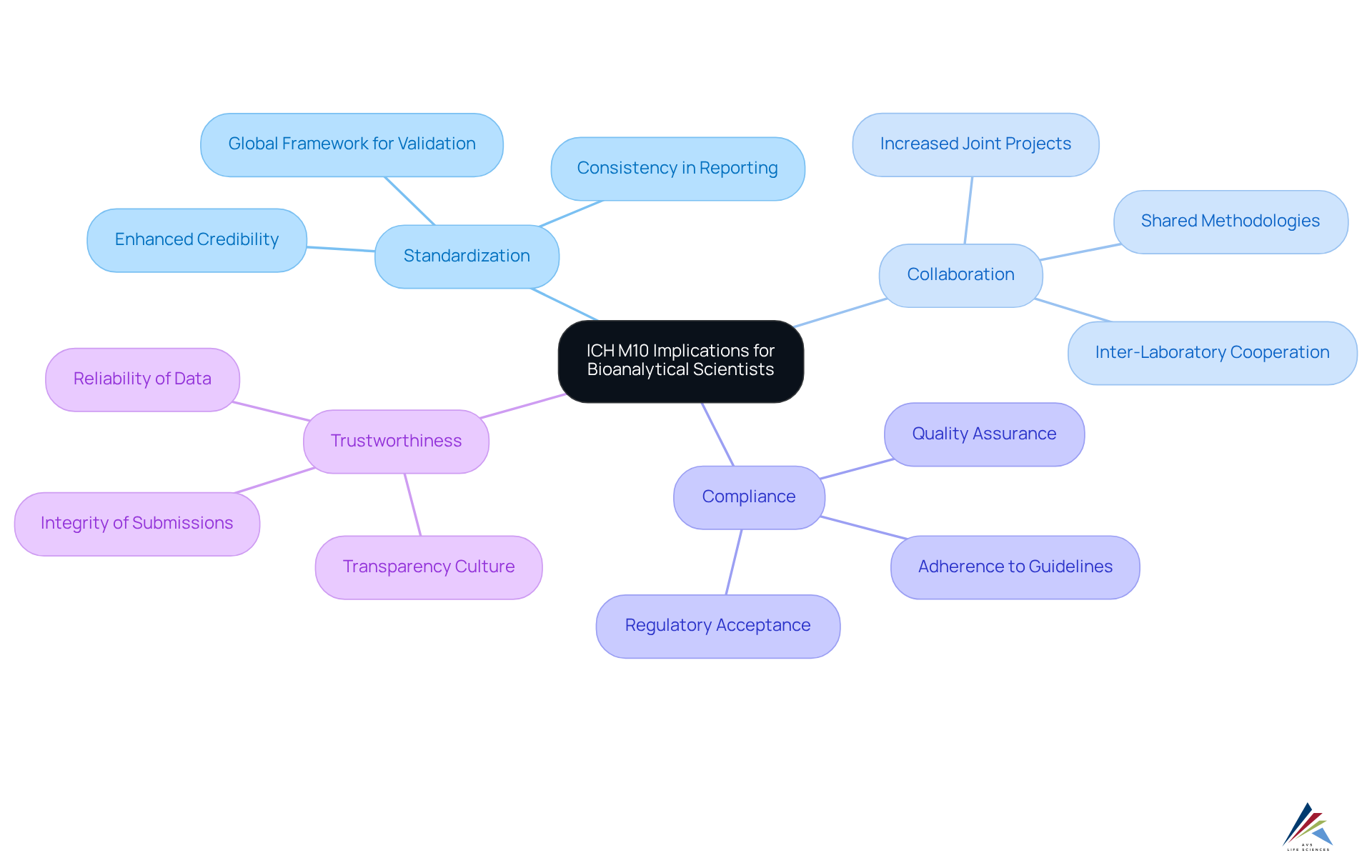
The implementation of ich m10 carries significant implications for bioanalytical scientists by establishing a standardized framework for method validation and sample analysis. Compliance with ich m10 guidelines not only enhances the credibility of bioanalytical results but also fosters greater collaboration and consistency across the industry. Notably, the adoption of ich m10 has led to a marked increase in inter-laboratory collaboration; studies indicate that bioanalytical scientists are now more inclined to engage in joint projects and share methodologies. This collaborative spirit is crucial as the regulatory landscape evolves, making it essential for adherence officers to remain informed about ich m10 guidelines. By ensuring compliance with these guidelines, ich m10, they contribute to advancing scientific practices in bioanalysis and uphold the integrity of regulatory submissions.
Furthermore, expert perspectives highlight the role of ich m10 in cultivating a culture of transparency and trustworthiness in bioanalytical work, thereby reinforcing ich m10's status as a cornerstone for adherence and quality assurance within the life sciences sector. At AVS Life Sciences, our extensive industry experience and comprehensive expertise in validation, quality compliance, and regulatory solutions empower compliance officers to navigate these guidelines adeptly, ensuring robust compliance and quality management in their operations.
Conclusion
The insights presented on ICH M10 underscore its pivotal role in standardizing bioanalytical method validation and ensuring regulatory compliance within the life sciences sector. By embracing these guidelines, compliance officers can significantly enhance the quality and reliability of bioanalytical data, which is essential for successful drug development and market approval.
Key arguments throughout the article emphasize the historical context of ICH M10, its global implementation, and the specific requirements for bioanalytical method validation, including:
- Precision
- Accuracy
- Selectivity
The discussion also highlights the importance of:
- Incurred Sample Reanalysis (ISR)
- Dilutional linearity
- Comprehensive documentation practices
All of these contribute to maintaining data integrity and fostering trust in bioanalytical results.
As the landscape of bioanalysis continues to evolve, adherence to ICH M10 guidelines will be paramount. Compliance officers are encouraged to remain informed and proactive in implementing these standards, ensuring their organizations not only meet regulatory expectations but also contribute to a culture of quality and transparency in the life sciences industry. Embracing these practices positions organizations for regulatory success and reinforces their commitment to advancing scientific excellence in bioanalysis.
Frequently Asked Questions
What services does AVS Life Sciences offer for ICH M10 compliance?
AVS Life Sciences provides a comprehensive suite of services to ensure adherence to ICH M10 standards, including dedicated support for API & Drug Product CMOs and Contract Test Labs, as well as expertise in quality management and regulatory standards.
Why were the ICH M10 guidelines established?
The ICH M10 guidelines were established to address discrepancies in bioanalytical method assessment across various regions, a necessity recognized by stakeholders since 2010. The finalized guidelines were adopted in May 2022 to harmonize global practices in bioanalytical method validation.
What is the significance of the ICH M10 guidelines for regulatory compliance?
Embracing ICH M10 is crucial for ensuring compliance and fostering trust in bioanalytical data, which is essential for drug development and regulatory submissions. Approximately 58% of organizations have begun implementing these guidelines, indicating a shift towards standardized practices.
What are the key requirements for bioanalytical method validation under ICH M10?
Key requirements include precision, accuracy, selectivity, sensitivity, and robustness. Compliance officers must ensure these methods are rigorously validated and documented, with Standard Operating Procedures (SOPs) created for all verification processes and outcomes.
How does AVS Life Sciences support GMP adherence and verification?
AVS Life Sciences offers expert solutions in GMP adherence, verification, and engineering to ensure organizations follow FDA regulations and GxP standards, enhancing compliance rates significantly.
What are some statistics related to ICH M10 compliance?
Studies show that methods adhering to ICH M10 verification parameters have a failure probability of less than 1% when the true total coefficients of variation (CV) are maintained at 10%. For example, an assay for levofloxacin achieved 99.06% with a % RSD value of 1.28%.
What is the importance of early communication with regulatory agencies during drug development?
Early communication with regulatory agencies is essential for ensuring adherence measures align with regulatory expectations, as highlighted in a recent 'Hot Topic' session discussing the challenges of implementing ICH M10.
How does AVS Life Sciences assist regulatory officers in navigating ICH M10 guidelines?
AVS Life Sciences leverages its extensive industry expertise to provide tailored verification, quality assurance, and regulatory solutions, helping regulatory officers efficiently navigate ICH M10 guidelines and meet stringent standards in the life sciences sector.
