10 Essential Features of a Clinical Trials Management System

Overview
The title "10 Essential Features of a Clinical Trials Management System" indicates that this article will delineate the critical functionalities required for the effective management of clinical trials. It underscores features such as:
- Robust data management
- Regulatory compliance
- User-friendly interfaces
- Real-time reporting
Each of these elements is vital for enhancing operational efficiency, ensuring adherence to regulations, and facilitating informed decision-making in the realm of clinical research.
Introduction
In the intricate realm of clinical research, the efficacy of a Clinical Trials Management System (CTMS) is pivotal to the success of a study. As the landscape evolves, the necessity for systems that not only streamline processes but also uphold compliance and data integrity becomes increasingly critical. This article examines ten fundamental features that characterize a robust CTMS, illustrating how these elements enhance research efficiency and regulatory adherence. Organizations often grapple with selecting the appropriate features—what challenges do they face, and how can they effectively leverage these tools to adeptly navigate the complexities of clinical trials?
AVS Life Sciences: Comprehensive Quality Compliance Features for CTMS
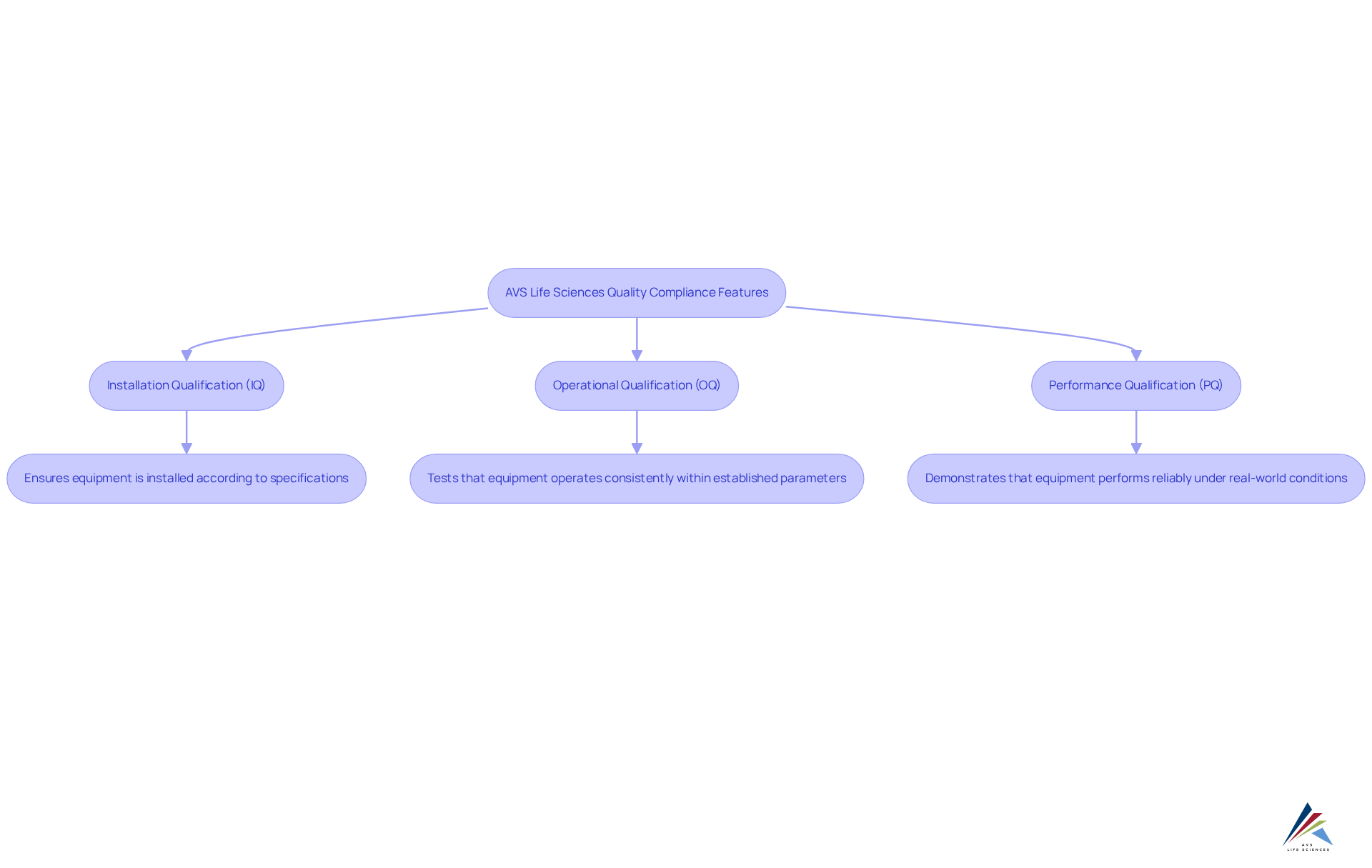
AVS Life Sciences addresses the pressing compliance challenges within the clinical trials management system by offering a comprehensive suite of quality compliance features. Their are essential for ensuring adherence to Good Manufacturing Practices (GMP) and other regulatory standards, thereby significantly enhancing the quality and reliability of research outcomes.
AVS's phase-appropriate quality strategies—including Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ)—empower research teams to adeptly navigate complex regulatory environments. Furthermore, extensive training programs ensure that personnel are thoroughly prepared to manage compliance-driven projects.
Notable implementations, such as those at Southern Illinois University School of Medicine, exemplify the effectiveness of these services in improving the efficiency of the clinical trials management system and ensuring regulatory compliance. By integrating these crucial attributes, AVS Life Sciences positions itself as a trusted ally in the evolving landscape of medical research.
Robust Data Management: Ensuring Accuracy and Integrity in Clinical Trials
A robust information management system within a clinical trials management system is essential for ensuring that all data collected during clinical studies is accurate, secure, and readily accessible. Key features, such as electronic information capture (EDC), facilitate real-time data validation and comprehensive audit trails, in alignment with GXP and FDA regulations. EDC systems have demonstrated the ability to reduce operational costs by as much as 30% while significantly lowering error rates, with pooled error rates for single-data entry methods at just 0.29% and double-data entry methods at 0.14%. By implementing rigorous information management protocols and adhering to Standard Operating Procedures (SOPs), organizations can bolster data integrity and ensure compliance with regulatory requirements, ultimately leading to more reliable experimental outcomes. The capacity to monitor patient progress and track adverse events in real time underscores the critical role of EDC in research studies, allowing for timely corrective actions and enhanced patient safety. This combination of features not only streamlines data collection but also elevates the overall quality of medical research.
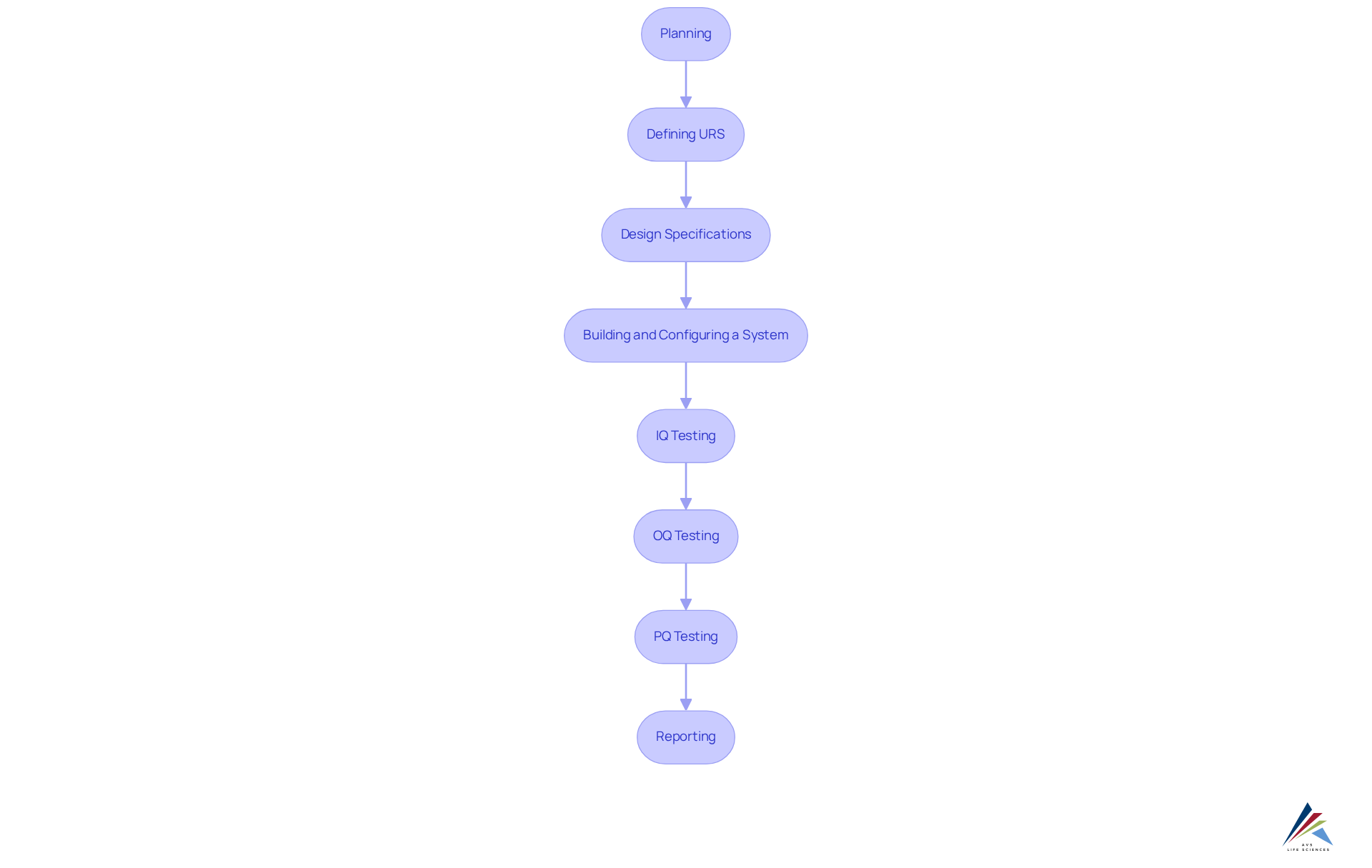
Stages of the Computer System Validation Process:
- Planning: Determine budget and timeline for validation.
- Defining URS: Outline the tasks the system must perform.
- Design Specifications: Decide on the system's functionality and appearance.
- Building and Configuring a System: Develop configured scripts for software design.
- IQ Testing: Execute scripts to verify installation methods.
- OQ Testing: Ensure the system operates as intended.
- PQ Testing: Simulate worst-case scenarios to validate performance.
- Reporting: Document validation results as evidence for system readiness.
This systematic approach to computer system validation reinforces the within clinical trials management systems, ensuring compliance and enhancing the quality of clinical research.
Regulatory Compliance: Essential Features for Meeting Industry Standards
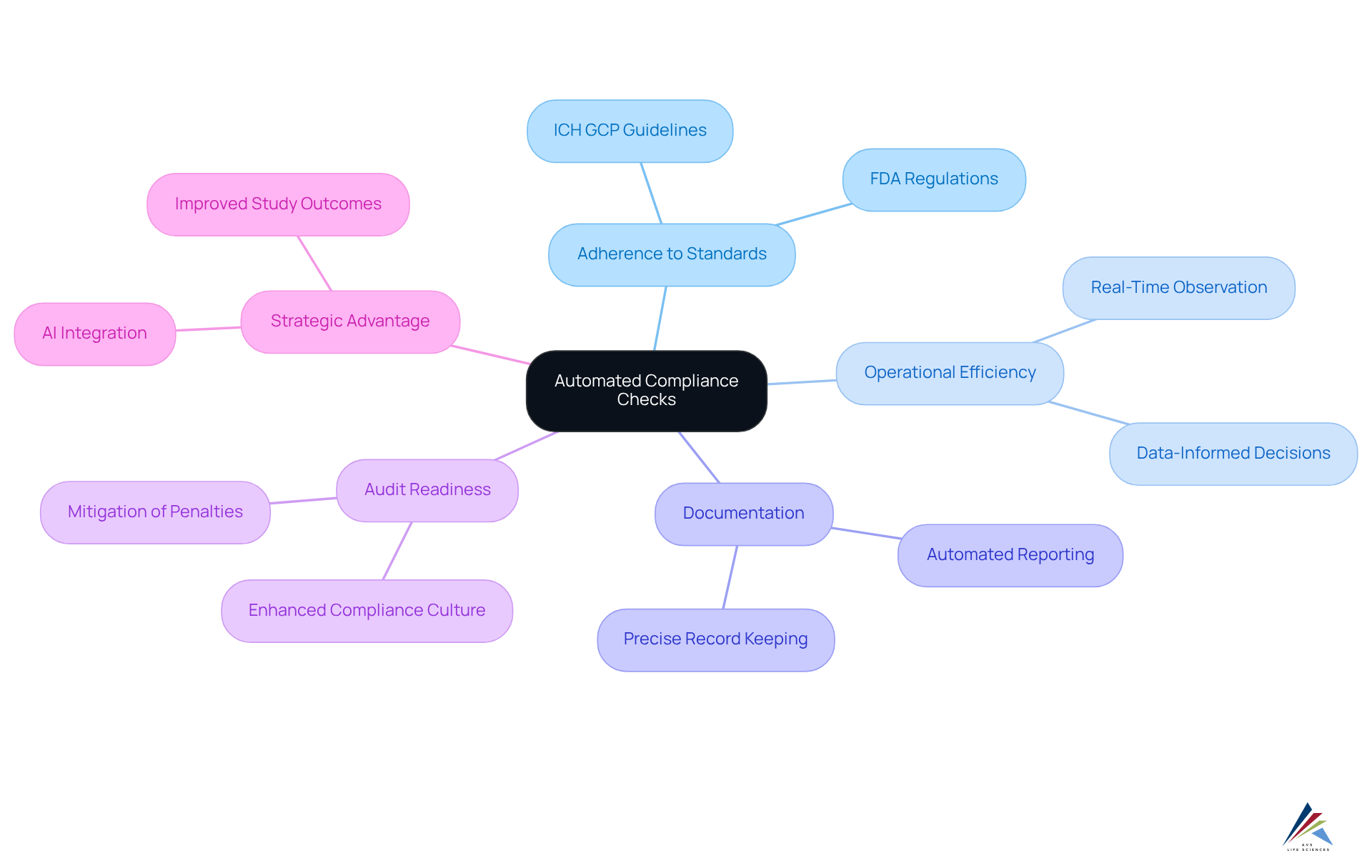
Automated compliance checks are a cornerstone feature of an effective clinical trials management system, essential for ensuring adherence to regulatory standards such as FDA regulations and ICH GCP guidelines. These automated systems facilitate real-time observation of testing activities, guaranteeing that all processes conform to established protocols and significantly reducing the risk of non-compliance. By integrating automated compliance checks, organizations can streamline the submission process, maintain precise documentation, and enhance audit readiness, thereby mitigating the potential for penalties associated with regulatory breaches.
The impact of these automated checks on testing efficiency is profound. They empower study managers to make rapid, data-informed decisions, identifying compliance concerns as they arise and enabling prompt corrective actions. For example, a clinical trials management system that is equipped with robust compliance features can track regulatory requirements and generate real-time reports, which are essential for timely submissions and audits. This proactive approach not only boosts but also cultivates a culture of compliance within the organization.
As we look towards 2025, the emphasis on automated compliance evaluations becomes increasingly critical, given the growing complexities of clinical studies. The integration of advanced technologies, such as AI and machine learning, further refines these compliance procedures, ensuring that assessments are executed with the highest standards of integrity and transparency. Industry specialists underscore that the automation of compliance checks transcends mere regulatory obligation; it represents a strategic advantage that can lead to improved study outcomes and accelerated access to life-saving therapies for patients.
User-Friendly Interface: Enhancing Usability for Clinical Trial Teams
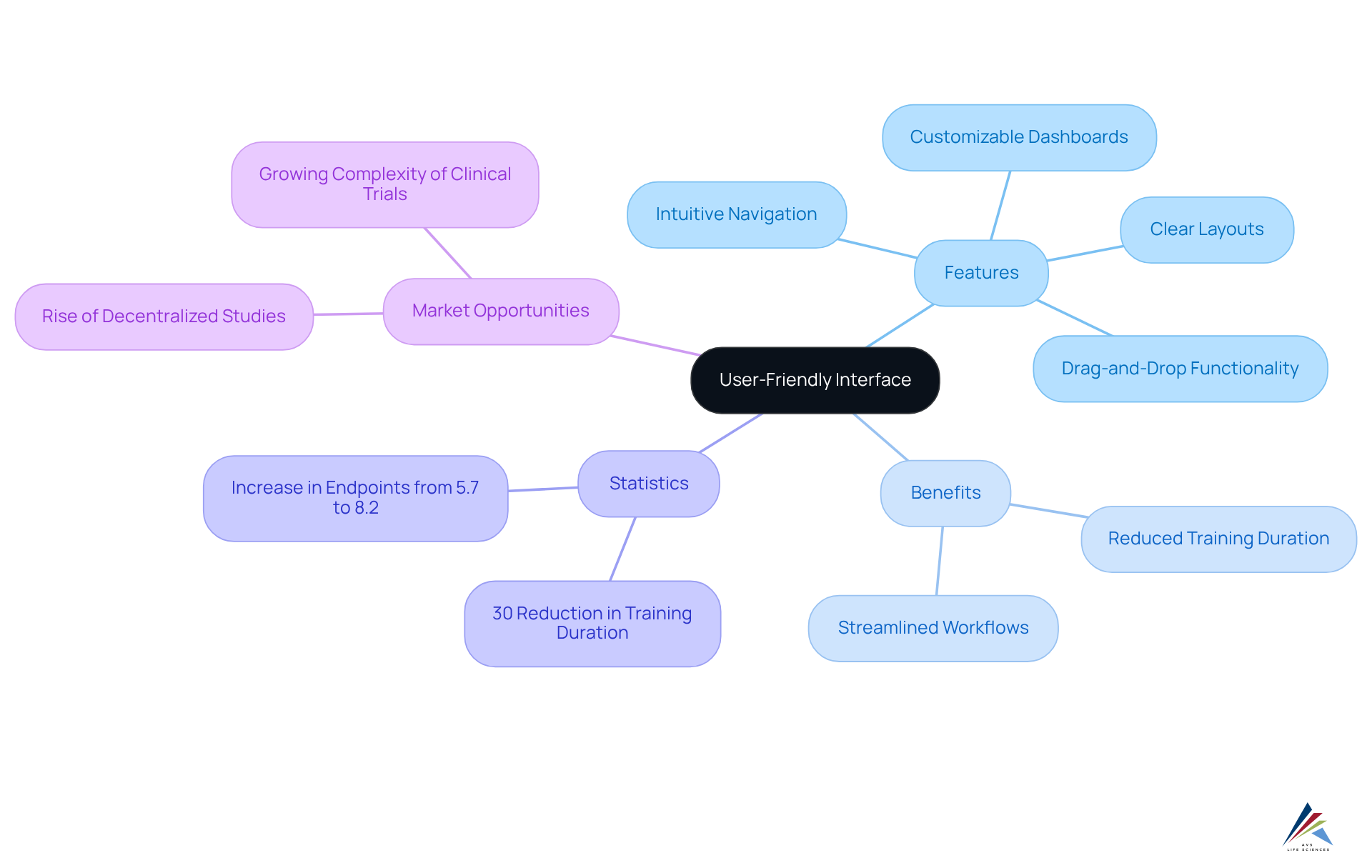
An easy-to-use interface in a clinical trials management system significantly enhances usability for research teams by offering intuitive navigation, clear layouts, and logical organization of information. Features such as customizable dashboards and drag-and-drop functionalities facilitate seamless access to critical data, streamlining workflows and minimizing the learning curve for new users.
Studies indicate that organizations prioritizing usability can reduce training duration by as much as 30%, allowing research teams to concentrate on their primary responsibilities without the disruption of complicated software.
The National Institutes of Health highlights the , reporting that the average number of endpoints in pivotal studies for new medications approved in 2022 was 8.2, up from 5.7 in 2012, marking a 44% increase in study complexity over the past decade. This trend underscores the imperative for intuitive design in CTMS platforms.
Furthermore, the rise of decentralized and virtual studies presents a significant opportunity for market expansion, making usability even more critical. By focusing on intuitive design, organizations can foster a more productive environment, ultimately enhancing the success of their clinical studies.
Integration Capabilities: Facilitating Seamless Data Flow Across Systems
The integration capabilities within a clinical trials management system are vital for facilitating seamless information flow across diverse systems, including electronic health records (EHR), laboratory information management systems (LIMS), and financial management tools. This not only ensures that information remains consistently updated and accessible across platforms but also significantly reduces the risk of errors, thereby enhancing overall process efficiency.
By enabling integration, organizations can foster improved collaboration among stakeholders, streamline research information management, and ultimately support more effective decision-making processes. The importance of interoperability is underscored by industry experts, who assert that a connected environment is crucial for maintaining information accuracy and compliance throughout the study lifecycle.
As the clinical trial landscape continues to evolve, the capacity to integrate various systems will be a key determinant of achieving successful outcomes. However, organizations must also address challenges such as information security concerns and system compatibility issues to fully harness the benefits of integration.
For example, the market for clinical trials management systems is projected to grow from $8.5 billion in 2025 to over $22.4 billion by 2033, highlighting the increasing importance of these solutions in the industry. Integrating expert insights and practical examples of seamless information flow can further enhance the effectiveness of clinical trials management systems in research.
Real-Time Reporting and Analytics: Driving Informed Decision-Making
Real-time reporting and analytics capabilities in a clinical trials management system empower research teams to make informed decisions based on current data. These capabilities facilitate the monitoring of , tracking of patient recruitment progress, and evaluation of milestones. By leveraging real-time insights, organizations can swiftly identify potential issues, adjust strategies as necessary, and enhance the overall effectiveness of the clinical trials management system.
Statistics indicate that timely insights play a crucial role in sustaining the momentum of medical studies and preventing costly setbacks, with a clinical trials management system facilitating systematic tracking of KPIs that leads to improved drug development outcomes. Furthermore, the use of a clinical trials management system for immediate access to patient information significantly boosts patient retention, greatly enhancing the likelihood of successful study completion.
As the clinical research landscape evolves in 2025, the focus on strategic KPIs—such as collaboration quality and proactive issue resolution—will be essential for fostering deeper alignment and trust among stakeholders. By integrating KPIs that evaluate collaboration quality, proactive issue resolution, and alignment with sponsor expectations, we can establish governance frameworks that strengthen partnerships, ultimately resulting in better outcomes.
Platforms like Suvoda provide real-time data access for analyzing study data, further supporting informed decision-making.
Scalability: Adapting to the Evolving Needs of Clinical Trials
A scalable clinical trials management system is essential for adapting to the evolving demands of clinical studies, empowering organizations to manage multiple projects simultaneously without sacrificing quality or compliance. The importance of modular design is paramount; it facilitates customizable workflows tailored to specific testing requirements.
For example, organizations can adopt features that enable flexible resource allocation, ensuring that teams can swiftly respond to shifting demands. Moreover, a robust clinical trials management system must adhere to regulatory compliance standards, including GXP and FDA regulations, to ensure data integrity and compliance with Good Documentation Practices (GDP).
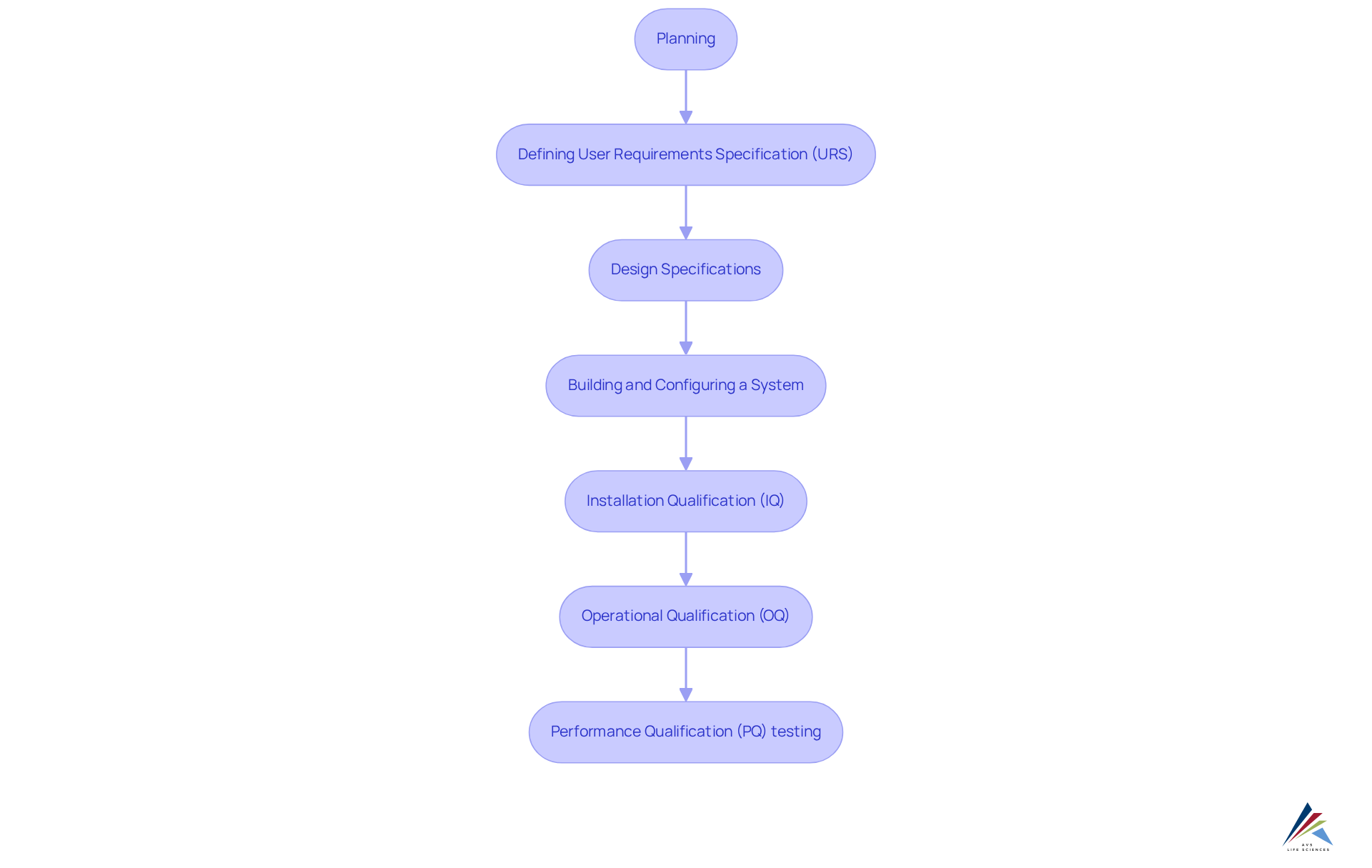
By investing in scalable solutions that integrate comprehensive quality management practices—encompassing the stages of computer system validation:
- Planning
- Defining User Requirements Specification (URS)
- Design Specifications
- Building and Configuring a System
- Installation Qualification (IQ)
- Operational Qualification (OQ)
- Performance Qualification (PQ) testing
clinical research teams position themselves to sustain operational effectiveness as study complexities escalate and regulatory landscapes transform. This adaptability not only enhances but also supports adherence to stringent regulatory standards, ultimately leading to more successful outcomes in research involving human subjects.
Training and Support: Ensuring Effective Implementation and User Adoption
The effective implementation of a clinical trials management system is fundamentally dependent on . These initiatives must include:
- Hands-on training sessions
- Detailed user manuals
- Ongoing technical assistance to address any challenges users may encounter
Research indicates that organizations providing thorough training resources can significantly bolster user confidence, with post-training assessments revealing an increase in comfort levels among research professionals. Notably, proficiency in recruitment and retention strategies improved markedly after training, with numerous participants reporting an enhanced capacity to navigate the complexities of medical studies.
Moreover, effective user adoption strategies for research software in 2025 underscore the necessity of customized training sessions tailored to the specific needs of research teams. Engaging training formats, such as:
- Interactive workshops
- Real-world simulations
have demonstrated efficacy in fostering a deeper understanding of the system's functionalities. Expert insights highlight the critical role of user manuals as essential resources that augment training initiatives, providing users with a reliable reference to optimize the system's capabilities. By prioritizing these components, organizations can ensure that their research teams are thoroughly equipped to leverage the full potential of their clinical trials management system, ultimately facilitating successful outcomes in their investigative endeavors.
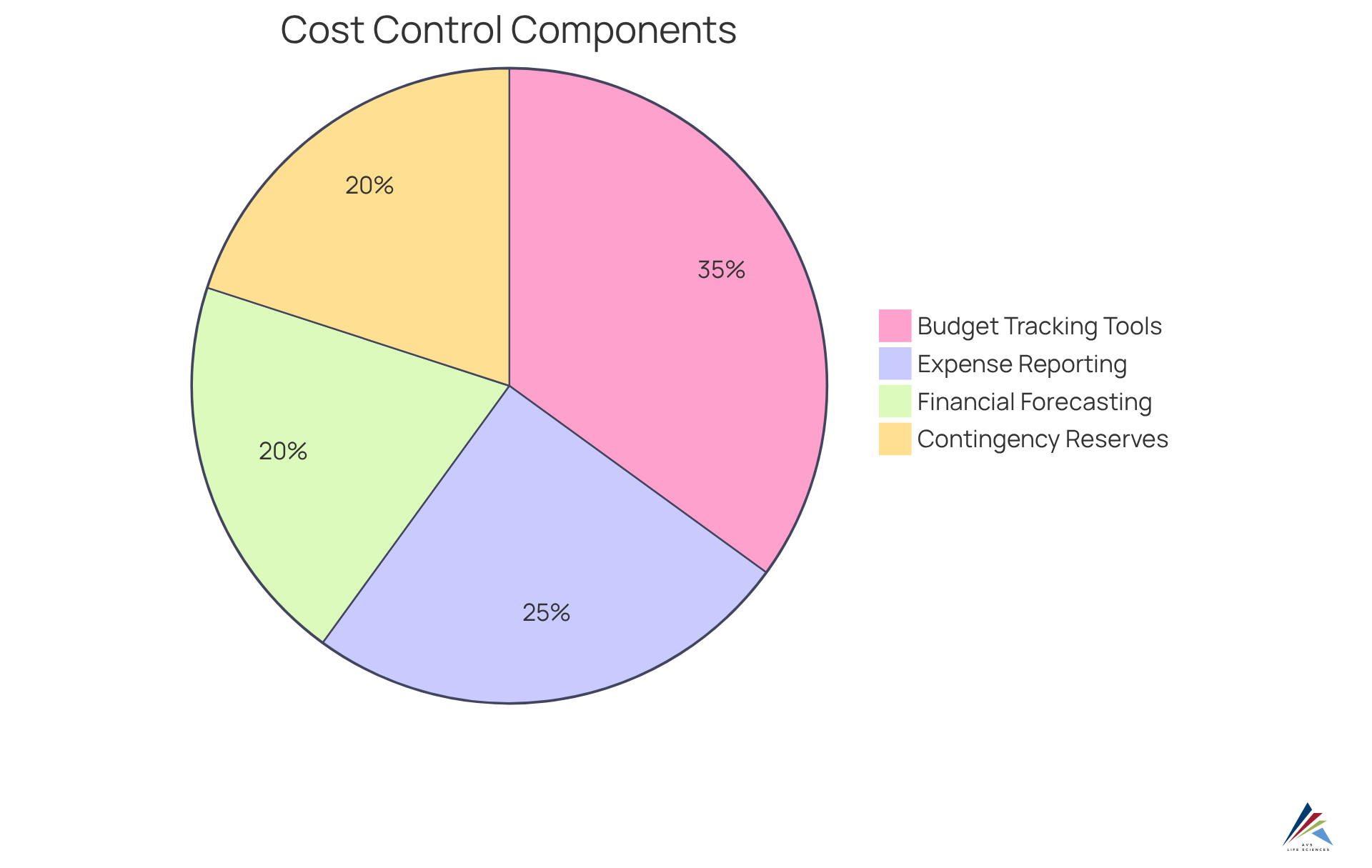
Cost Control: Managing Budgets Effectively in Clinical Trials
Cost control features within a clinical trials management system are essential for effectively managing budgets throughout the clinical research lifecycle. Key components, including budget tracking tools, expense reporting, and financial forecasting capabilities, empower organizations to minimize financial risks and ensure efficient resource allocation. For example, real-time budget tracking systems can identify potential overruns early by monitoring expenses against the budget at both site and patient levels, fostering transparency among stakeholders. This proactive is crucial; studies indicate that "Seventy percentage of RCTs exceeded their budget by more than 50%", often due to slow recruitment and unexpected protocol changes.
The significance of budget tracking tools is underscored by their ability to enhance success rates. Efficient financial management transforms budgeting from a compliance requirement into a strategic advantage, thereby improving the chances of success by ensuring that sufficient resources are available. Furthermore, scenario planning with rolling forecasts enables finance teams to anticipate changes and adjust budgets accordingly, which is vital in a landscape where "the average total cost of developing a new marketable treatment is estimated to be $2B".
In 2025, the integration of sophisticated budget monitoring tools within the clinical trials management system will be imperative for organizations aiming to navigate the complexities of research studies. By leveraging these tools, sponsors can align financial data with healthcare operations, ultimately leading to improved outcomes and adherence to funding requirements. As the sector evolves, the emphasis on robust cost management attributes will remain a critical factor in the success of research studies. Additionally, establishing appropriate contingency reserves of 10-15% of the overall budget is essential for managing budget risk in research studies, further highlighting the strategic importance of financial planning in this domain.
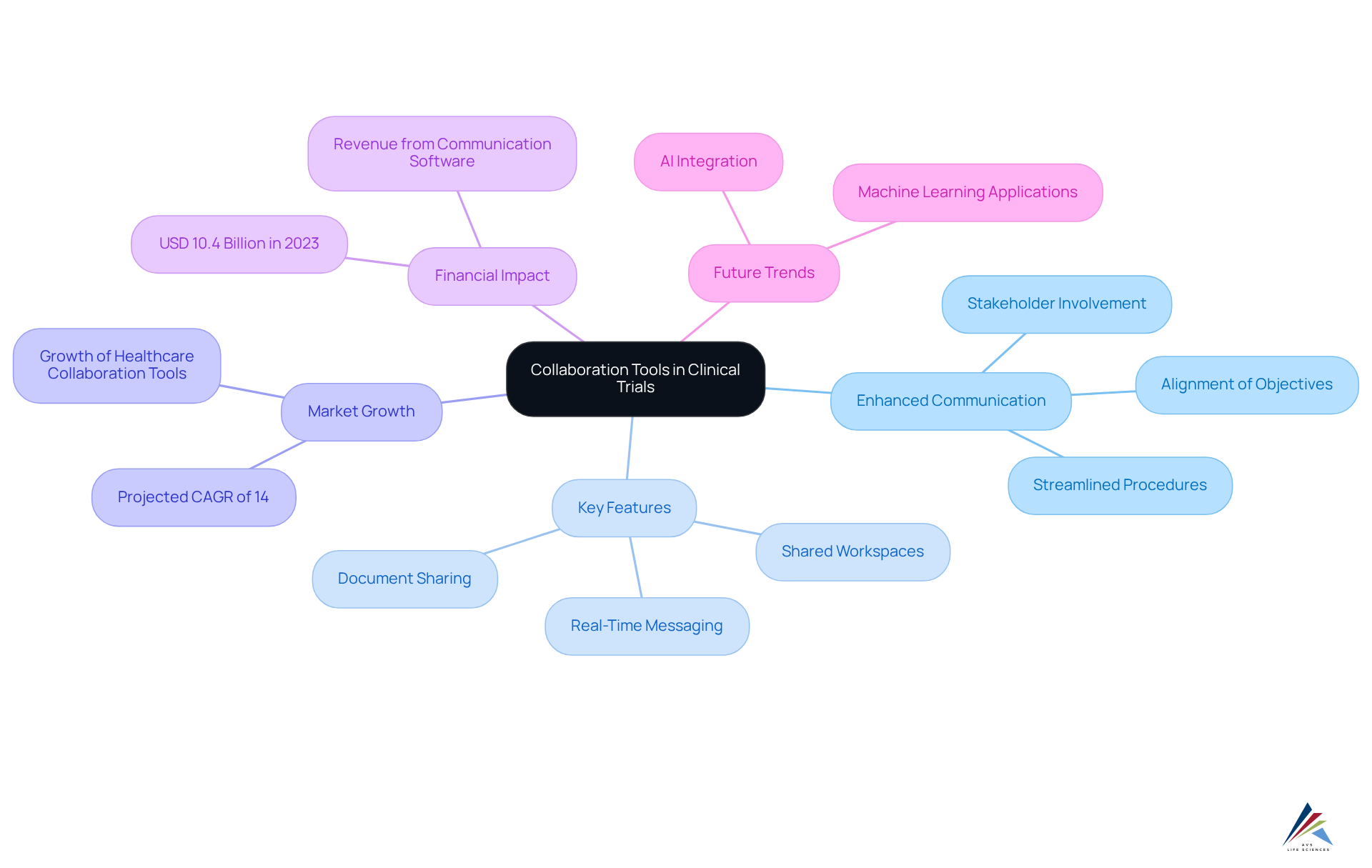
Collaboration Tools: Enhancing Communication Among Clinical Trial Stakeholders
Collaboration tools integrated into a clinical trials management system significantly enhance communication among research stakeholders, including sponsors, researchers, and site personnel. Features such as shared workspaces, real-time messaging, and document sharing capabilities facilitate seamless collaboration and information exchange. This is particularly crucial as the is projected to expand at a compound annual growth rate (CAGR) of 14% by 2026, highlighting the increasing complexity of studies and the necessity for efficient management solutions.
Effective communication fosters stakeholder involvement, streamlines procedures, and ensures alignment among all parties working towards common objectives. For example, the communication and coordination software segment generated USD 10.4 billion in 2023, underscoring the demand for tools that enhance collaboration among healthcare providers. Moreover, organizations that leverage these tools can expect improved operational efficiencies, as evidenced by the rising adoption of the clinical trials management system (CTMS) solutions, which are essential for managing complex studies.
As medical studies become more intricate, with larger patient populations and diverse treatment methodologies, the necessity for robust communication tools becomes increasingly apparent. By harnessing advanced technologies such as AI and machine learning, organizations can further refine their communication strategies, ensuring that all stakeholders remain informed and engaged throughout the project lifecycle. This commitment to effective communication not only enhances trial outcomes but also positions organizations to adeptly navigate the evolving landscape of clinical research.
Conclusion
The exploration of essential features within a clinical trials management system (CTMS) underscores the critical role these components play in enhancing the efficiency, compliance, and overall success of clinical research. By integrating robust data management, regulatory compliance, user-friendly interfaces, and advanced collaboration tools, organizations can significantly improve their operational workflows and research outcomes.
Key insights from the article highlight the importance of quality compliance features, such as those offered by AVS Life Sciences, which ensure adherence to regulatory standards and enhance the reliability of research data. Furthermore, the implementation of real-time reporting and analytics enables research teams to make informed decisions, while scalable solutions allow organizations to adapt to the evolving demands of clinical trials. The emphasis on training and support ensures that users can effectively navigate these systems, ultimately leading to better project management and successful study completion.
As the landscape of clinical trials continues to grow in complexity, the adoption of comprehensive CTMS solutions will be paramount. Organizations are encouraged to prioritize these essential features to not only meet regulatory requirements but also to foster a culture of compliance and collaboration. By doing so, they can pave the way for more efficient clinical trials, ultimately accelerating the development of life-saving therapies and improving patient outcomes.
Frequently Asked Questions
What compliance challenges does AVS Life Sciences address in clinical trials management systems?
AVS Life Sciences addresses compliance challenges by offering a comprehensive suite of quality compliance features, including validation and commissioning services to ensure adherence to Good Manufacturing Practices (GMP) and other regulatory standards.
What are the phase-appropriate quality strategies provided by AVS Life Sciences?
AVS Life Sciences provides Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) as part of their phase-appropriate quality strategies to help research teams navigate complex regulatory environments.
How does AVS Life Sciences support personnel in managing compliance-driven projects?
AVS Life Sciences offers extensive training programs to ensure that personnel are thoroughly prepared to manage compliance-driven projects effectively.
Can you provide an example of AVS Life Sciences' effectiveness in improving clinical trials management?
An example of AVS Life Sciences' effectiveness is its implementation at Southern Illinois University School of Medicine, which improved the efficiency of the clinical trials management system and ensured regulatory compliance.
What is the importance of a robust information management system in clinical trials?
A robust information management system is essential for ensuring that data collected during clinical studies is accurate, secure, and readily accessible, ultimately leading to more reliable experimental outcomes.
What key features are included in electronic data capture (EDC) systems?
EDC systems include real-time data validation, comprehensive audit trails, and adherence to GXP and FDA regulations, which help reduce operational costs and error rates.
What is the significance of monitoring patient progress and tracking adverse events in real time?
Monitoring patient progress and tracking adverse events in real time allows for timely corrective actions and enhances patient safety during research studies.
What are the stages of the Computer System Validation Process?
The stages include Planning, Defining User Requirements Specification (URS), Design Specifications, Building and Configuring a System, IQ Testing, OQ Testing, PQ Testing, and Reporting.
How do automated compliance checks benefit clinical trials management systems?
Automated compliance checks ensure adherence to regulatory standards, facilitate real-time observation of testing activities, streamline submission processes, and enhance audit readiness, reducing the risk of non-compliance.
What advancements are expected in automated compliance evaluations by 2025?
By 2025, the integration of advanced technologies such as AI and machine learning is expected to refine compliance procedures, ensuring assessments are executed with high standards of integrity and transparency.
Why is the automation of compliance checks considered a strategic advantage?
The automation of compliance checks is considered a strategic advantage because it improves operational efficiency, enables rapid data-informed decisions, and can lead to better study outcomes and faster access to life-saving therapies for patients.
