10 Key Benefits of Healthcare Compliance Consulting for Pharma

Overview
The key benefits of healthcare compliance consulting for pharmaceutical companies are significant:
- Enhanced operational efficiency
- Improved risk management
- Establishment of robust regulatory relationships
Pharmaceutical companies face numerous compliance challenges that can hinder their success. AVS Life Sciences addresses these challenges by offering tailored consulting services designed to streamline processes, mitigate compliance risks, and foster trust with regulatory authorities. This not only leads to better quality management but also reduces the penalties associated with non-compliance. By partnering with AVS Life Sciences, companies can navigate the complexities of healthcare regulations effectively, ensuring they remain compliant and competitive in the market.
Introduction
Navigating the intricate landscape of pharmaceutical regulations presents a formidable challenge for many companies, with stakes that extend beyond financial implications to ethical considerations. Healthcare compliance consulting emerges as a vital ally, equipping pharmaceutical firms with the expertise essential for enhancing operational efficiency, mitigating risks, and upholding the highest quality standards. Yet, as regulations evolve at a rapid pace, the pressing question remains: how can organizations ensure they are not merely compliant but also thriving? This article explores ten key benefits of healthcare compliance consulting, illustrating how strategic partnerships empower pharmaceutical companies to adeptly navigate complexities while fostering innovation and integrity.
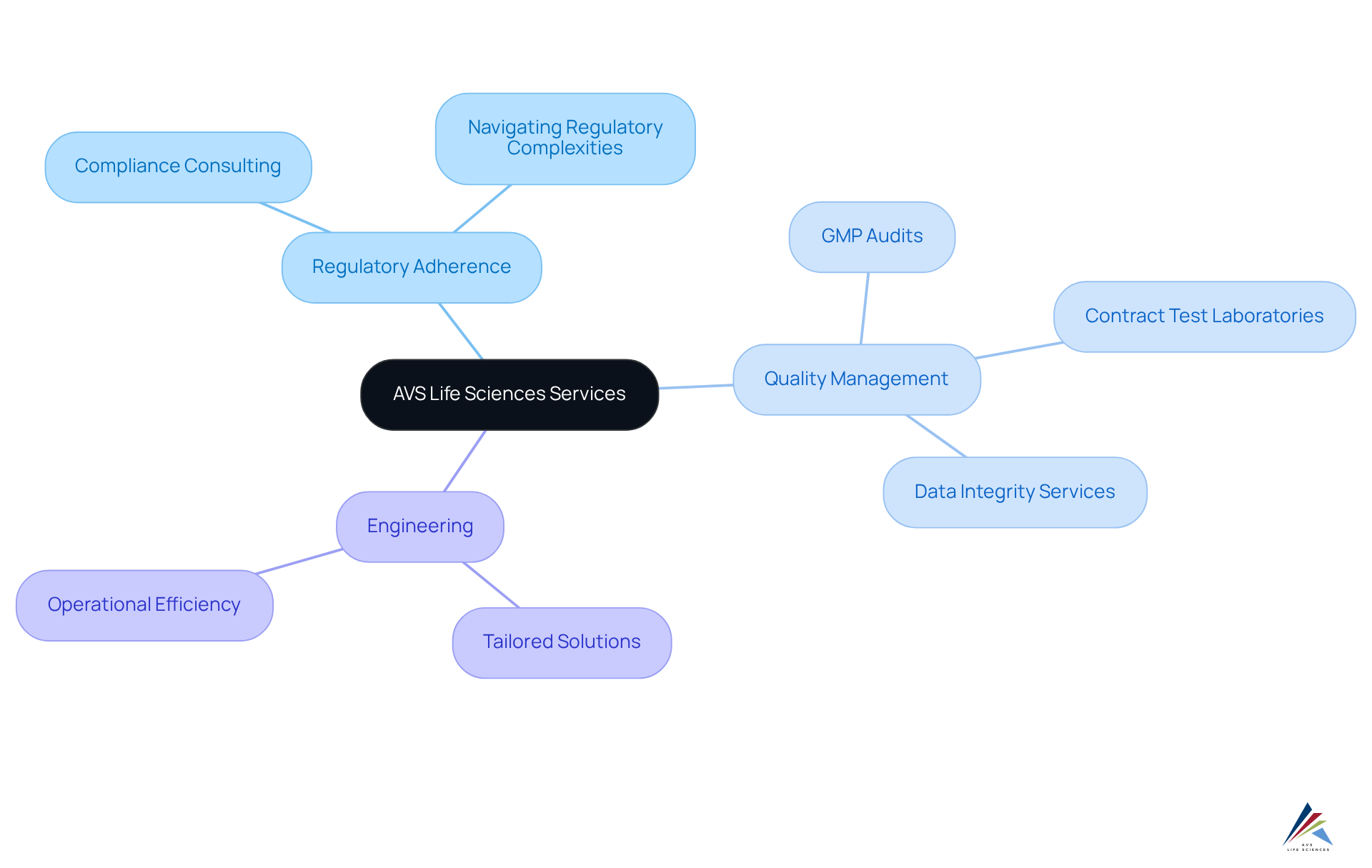
AVS Life Sciences: Comprehensive Regulatory and Quality Solutions for Pharmaceutical Companies
AVS Life Sciences offers a comprehensive suite of services tailored to the distinct needs of pharmaceutical firms, with a strong emphasis on regulatory adherence, quality management, and engineering. As the pharmaceutical industry grapples with stringent regulations and oversight, AVS empowers organizations to effectively navigate these complexities. Current trends indicate that 70% of pharmaceutical firms are increasingly outsourcing R&D to enhance efficiency and access specialized expertise, underscoring the critical role of quality management in ensuring compliance and fostering innovation.
By providing customized solutions, such as:
AVS enables clients to uphold high standards of quality through healthcare compliance consulting throughout the product lifecycle. This commitment is essential, as non-compliance can incur , including billions in fines for off-label promotion. AVS's expertise guarantees that organizations can implement effective quality management strategies, which are vital for mitigating risks associated with regulatory scrutiny.
The company's strategy not only fosters a culture of compliance but also enhances operational efficiency, positioning AVS as a reliable partner in the pharmaceutical sector. Their extensive experience and proven track record in delivering tailored consulting services establish them as leaders in navigating the ever-evolving landscape of pharmaceutical regulations.
Enhanced Risk Management: Safeguarding Pharmaceutical Operations
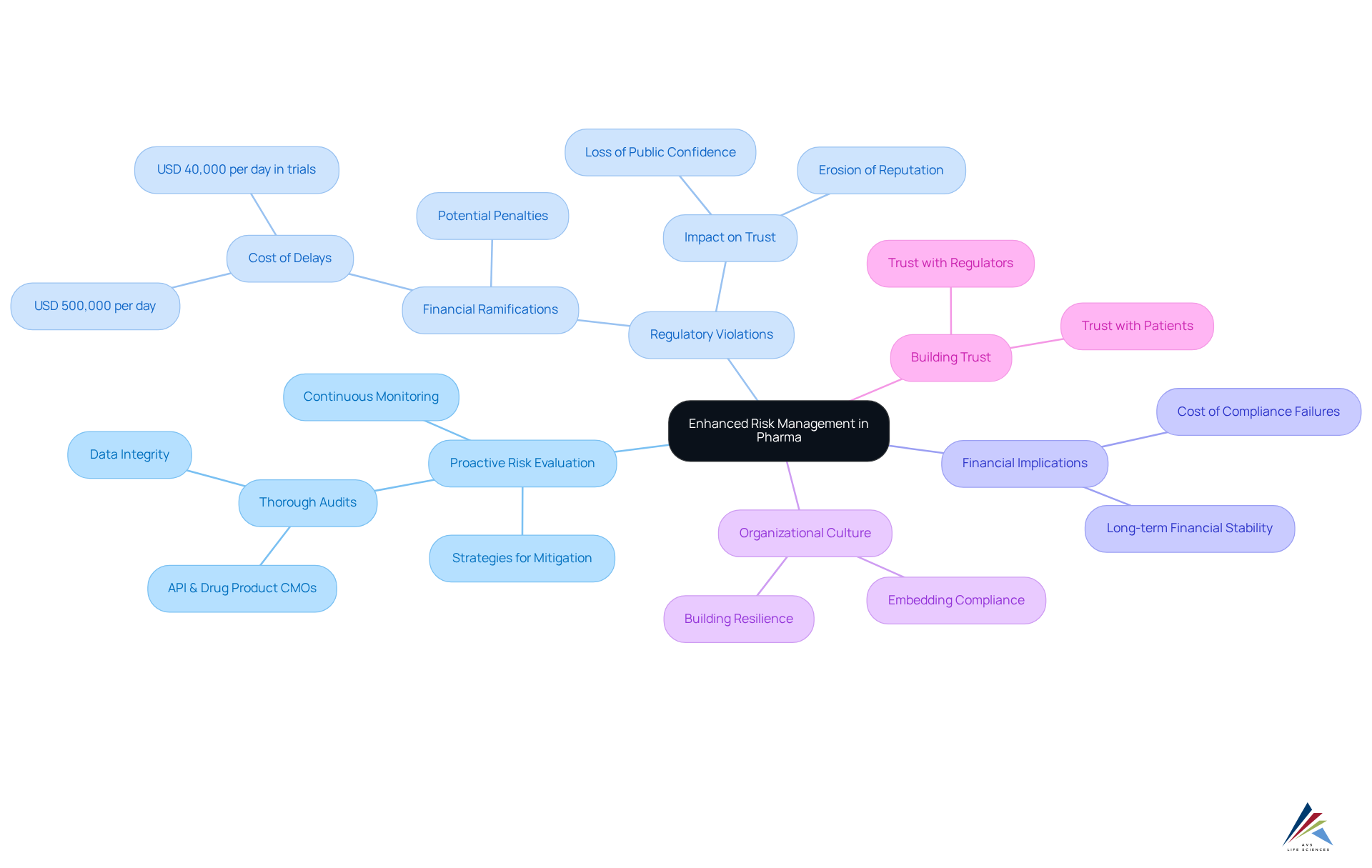
Healthcare compliance consulting plays a pivotal role in enhancing risk management by identifying vulnerabilities within pharmaceutical operations. AVS Life Sciences adopts a proactive risk evaluation method, equipping clients with strategies designed to mitigate potential regulatory violations. This forward-thinking approach not only but also ensures the safety and efficacy of pharmaceutical products.
Recent regulatory violations in the pharmaceutical sector have underscored the financial ramifications of inadequate risk management. A single regulatory misstep, for example, can result in delays costing upwards of USD 500,000 in lost drug sales per day, in addition to possible penalties. Such instances highlight the urgent need for robust regulatory frameworks that emphasize transparency and accountability.
Proactive risk assessment transcends mere defense; it is a strategic necessity. By embedding adherence into the organizational culture, companies can cultivate resilience against avoidable risks and costs. AVS Life Sciences identifies vulnerabilities through thorough audits—including API & Drug Product CMOs, Data Integrity, and continuous monitoring—ensuring that clients stay ahead of regulatory demands. This strategy builds trust with regulators and patients alike, ultimately enhancing the company's reputation and market standing.
The repercussions of regulatory violations extend beyond immediate financial setbacks; they can erode public confidence and lead to enduring reputational damage. Organizations that view adherence to regulations as fundamental are better positioned to navigate the complexities of the pharmaceutical landscape with healthcare compliance consulting, ensuring sustained operational success and patient safety.
Operational Efficiency: Streamlining Processes Through Compliance Consulting
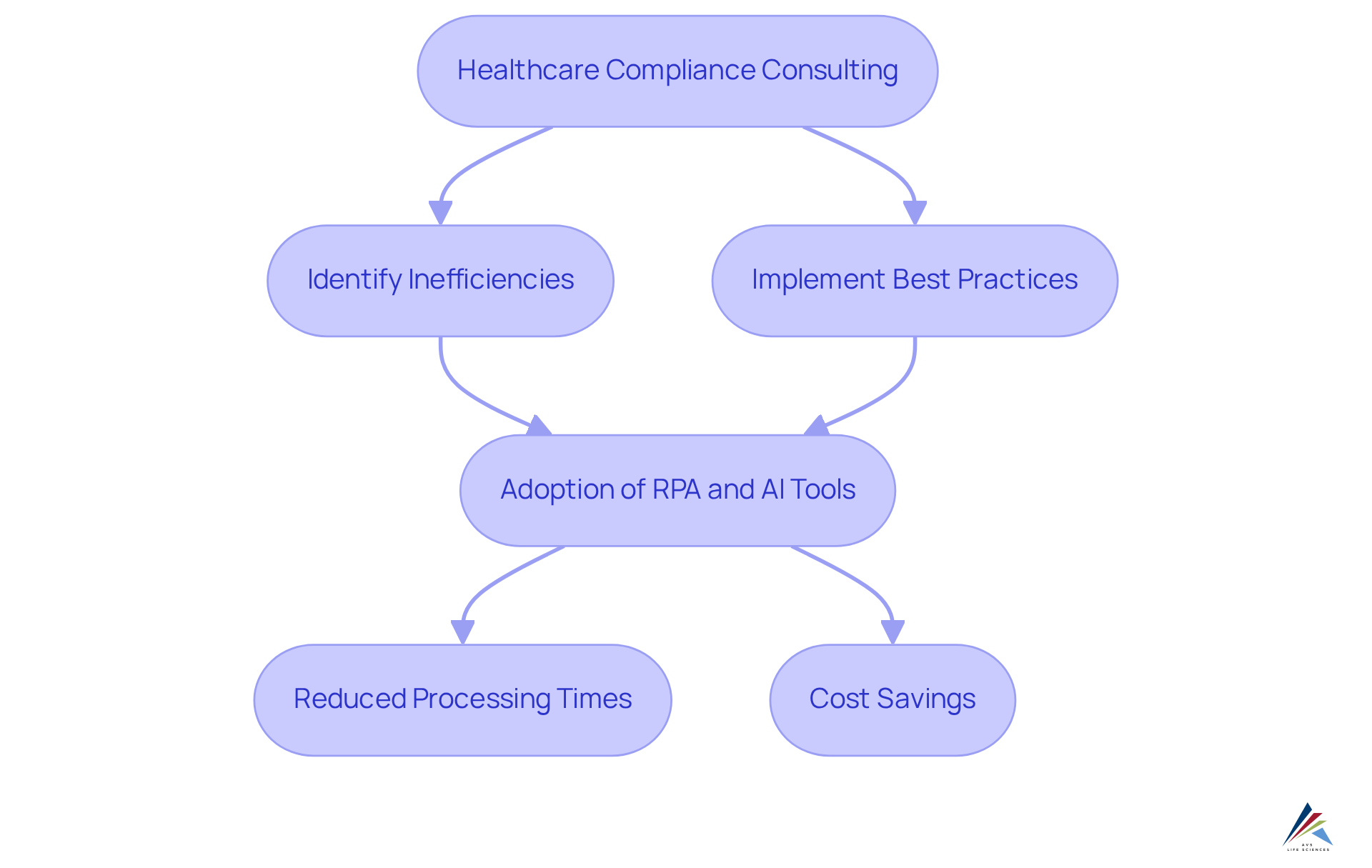
Healthcare compliance consulting plays a pivotal role in assisting pharmaceutical companies by streamlining their operations, identifying inefficiencies, and implementing industry-tailored best practices. AVS Health Sciences engages in healthcare compliance consulting by collaborating closely with clients to enhance processes, ensuring regulatory requirements are met without sacrificing productivity. This focus on operational efficiency, supported by , not only saves time but also significantly reduces expenses associated with regulatory failures.
For instance, AVS Life Sciences successfully aided a leading biotechnology company in upgrading their manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility, completing the project on schedule and within budget. This partnership allowed the client to concentrate on developing medicines while AVS ensured full traceability through meticulous documentation practices.
Furthermore, pharmaceutical companies that utilize healthcare compliance consulting and adopt Robotic Process Automation (RPA) report a notable improvement in adherence to regulations and an increase in productivity, with specific studies indicating that RPA can reduce processing times in areas such as insurance verification by as much as 90%. Companies utilizing AI-driven automation tools within the pharmaceutical sector can achieve substantial cost savings, with some reports suggesting savings of up to 300% in the first year of implementation.
As emphasized by industry leaders, 'Effective adherence is not just about control; it’s about fostering an environment where engagement thrives,' highlighting the balance necessary for effective process management. By implementing these strategies, including healthcare compliance consulting and the adoption of digitized batch processing solutions, pharmaceutical firms can enhance their operational frameworks, ultimately leading to improved adherence outcomes and better financial performance.
Expert Guidance: Navigating Complex Regulatory Landscapes
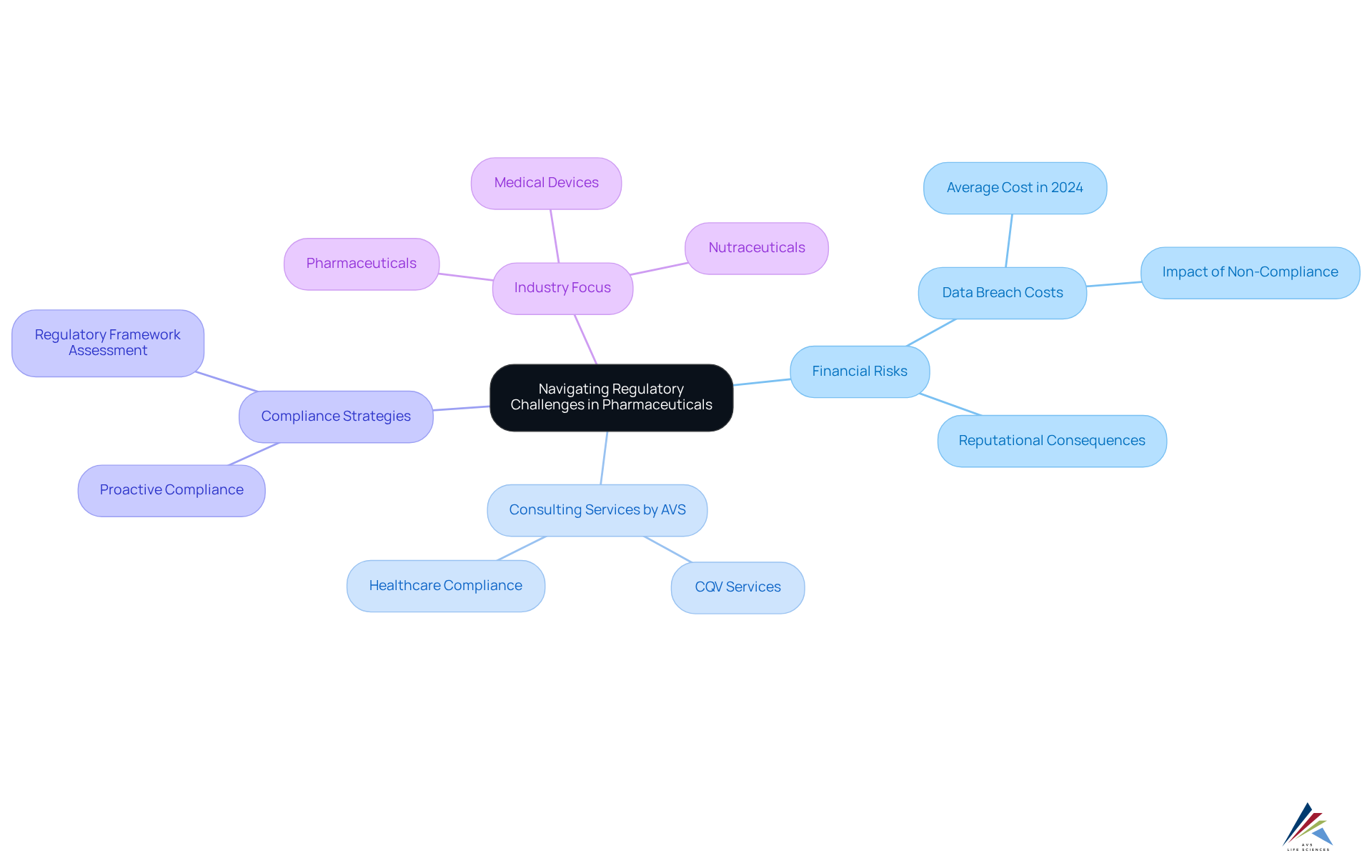
Navigating the complex legal framework of the pharmaceutical industry presents significant challenges for organizations. With a multitude of regulations in place, the risk of non-compliance is substantial, potentially leading to financial losses averaging $4.88 million per data breach in 2024. AVS Life Sciences stands as your committed partner for comprehensive regulatory, adherence, and quality solutions, specializing in healthcare compliance consulting to help clients navigate these complexities and develop strategies that align with regulatory mandates across biopharmaceuticals, medical devices, and nutraceuticals. This proactive support is essential in , which can result in serious financial and reputational consequences.
Industry specialists emphasize that successful regulatory strategies not only ensure compliance with rules but also enhance operational efficiency and foster a culture of ethical decision-making. By leveraging AVS Life Sciences' extensive healthcare compliance consulting services, including CQV (Commissioning, Qualification, and Validation), clients can effectively navigate the compliance landscape, safeguarding their interests and promoting sustainable growth in an evolving environment. Furthermore, 61% of corporate risk and regulatory professionals have identified keeping up with regulatory changes as their primary strategic focus over the next 12 to 18 months, underscoring the importance of proactive regulatory strategies.
To achieve success, organizations must regularly assess their regulatory frameworks and adapt to new rules. This approach not only reinforces compliance but also positions them favorably within the industry, ensuring they remain ahead of the curve in a rapidly changing regulatory landscape.
Comprehensive Training Programs: Empowering Staff with Compliance Knowledge
AVS Life Sciences offers comprehensive training programs designed to equip staff with the essential knowledge required to uphold regulatory standards, particularly in relation to Good Manufacturing Practices (GMP) audits. These programs cover critical topics such as:
These topics are specifically tailored for:
- API and drug product manufacturers
- Contract test laboratories
- Other relevant facilities
Research demonstrates that significantly diminishes errors and contamination risks, thereby ensuring a consistent, high-quality end product. Organizations prioritizing GMP training have reported notable improvements in product quality and regulatory compliance, especially within their manufacturing, storage, and distribution processes.
Furthermore, ongoing training is crucial for keeping employees informed about compliance changes, an essential factor in a rapidly evolving industry. By investing in thorough training, organizations cultivate a culture of healthcare compliance consulting, which enhances operational integrity and ultimately leads to improved outcomes and reduced risks associated with non-compliance.
AVS Life Sciences stands out as a leading provider of quality management and compliance solutions, transforming GMP facilities and ensuring adherence to quality standards for biotechnology clients.
Quality Assurance: Maintaining High Standards in Pharmaceutical Products
Quality assurance is fundamental to the pharmaceutical industry, safeguarding consumer safety and ensuring that products adhere to stringent standards of efficacy and safety. AVS Health Sciences plays a crucial role in assisting clients in creating thorough quality assurance frameworks that comply with legal requirements and promote a culture of excellence within organizations. This dedication to quality mitigates risks associated with product recalls—96% of organizations reported experiencing a recall in the past five years, according to Chris Nahil—and bolsters the organization's reputation in a competitive marketplace.
By implementing best practices in quality management, such as proactive risk management and continuous improvement models, AVS Sciences empowers clients to maintain high standards in their pharmaceutical products, ultimately enhancing consumer trust and satisfaction. Recent trends indicate that organizations investing in quality management systems (QMS) are better positioned to recover from product recalls, with 83% of respondents acknowledging that a QMS solution facilitated their recovery efforts, as noted by ETQ.
Through customized , including extensive GXP compliance services and validation solutions, AVS Biosciences ensures that clients are prepared to navigate the intricacies of quality assurance. A significant example of this commitment is demonstrated in a recent case study where AVS Sciences supported a leading biotechnology company in enhancing their manufacturing area from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. This project was completed on schedule and within budget, demonstrating AVS's expertise in quality compliance and regulatory standards.
By capturing important lessons learned during this process, AVS Sciences not only reinforced their client's quality assurance practices but also fostered a collaborative environment focused on continuous improvement. This strengthens AVS Sciences' dedication to consumer safety and product integrity.
Ethical Practices: Fostering a Culture of Compliance and Integrity
Nurturing a culture of adherence and integrity is crucial for pharmaceutical firms, and AVS Sciences plays a pivotal role in this transformation. By emphasizing ethical practices in its consulting services, AVS Sciences aids organizations in establishing robust policies and procedures that promote transparency and accountability. This ethical foundation cultivates trust among clients, regulators, and the public. As Stephen Covey aptly states, 'Moral authority comes from following universal and timeless principles like honesty and integrity.' Such principles are essential in the life sciences sector, where the stakes are high, and the need for ethical conduct is paramount.
Instances of ethical practices in healthcare compliance consulting include the implementation of comprehensive training programs, such as GXP Training, that educate employees on regulatory requirements and ethical standards. AVS Life Sciences offers healthcare compliance consulting to ensure that its clients are well-prepared to navigate intricate regulatory environments, thereby strengthening a culture of integrity within their organizations. The impact of a robust regulatory culture is significant; it not only mitigates risks but also enhances the overall reputation of pharmaceutical firms. By prioritizing integrity and developing Quality Management Systems, organizations can cultivate a sustainable environment that supports ethical decision-making and long-term success in the industry. To implement these practices effectively, companies should regularly and engage in continuous improvement initiatives.
Improved Documentation: Ensuring Transparency and Accountability
Effective documentation practices are essential for ensuring transparency and accountability within pharmaceutical operations. AVS Life Sciences excels in developing comprehensive documentation systems specifically designed to meet rigorous regulatory requirements. These systems not only streamline but also serve as critical tools for internal regulatory initiatives.
Notably, statistics reveal that a substantial percentage of audit failures result from insufficient documentation, highlighting the imperative for meticulous record-keeping. By implementing robust documentation strategies, organizations can enhance their regulatory posture, mitigate risks, and foster a culture of accountability.
AVS Life Sciences leverages its expertise to support clients in establishing documentation frameworks that align with industry standards, ensuring they are thoroughly prepared for regulatory scrutiny and capable of maintaining high-quality operations throughout the product lifecycle.
Proactive Compliance Strategies: Anticipating and Mitigating Risks
Proactive regulatory strategies are essential for anticipating and mitigating risks within the pharmaceutical industry. AVS Life Sciences collaborates with clients to identify potential regulatory challenges and develop strategies that address these issues before they escalate. This forward-thinking approach not only safeguards organizations from regulatory penalties but also enhances overall operational resilience.
Organizations that adopt ongoing adherence practices report a significant decrease in incidents related to regulations, with 91% intending to implement such strategies within the next five years. Furthermore, a proactive stance can elevate a company's reputation, fostering trust among stakeholders. As Rachel Cohn aptly states, 'The reward is in the risk,' underscoring the importance of taking calculated risks in regulatory management.
By anticipating adherence risks, particularly in the evolving landscape of 2025, AVS Life Sciences equips its clients to navigate legal complexities efficiently through comprehensive GMP audit services. These include evaluations of:
- API and drug product CMOs
- Contract testing laboratories
- Production facilities
A notable instance of this is AVS's successful upgrade of a biotechnology GMP facility, where they assisted a leading San Francisco-based biotechnology company in transitioning from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. This partnership ensured compliance with stringent and allowed the client to focus on developing innovative medicines, ultimately enhancing their operational success in a competitive market.
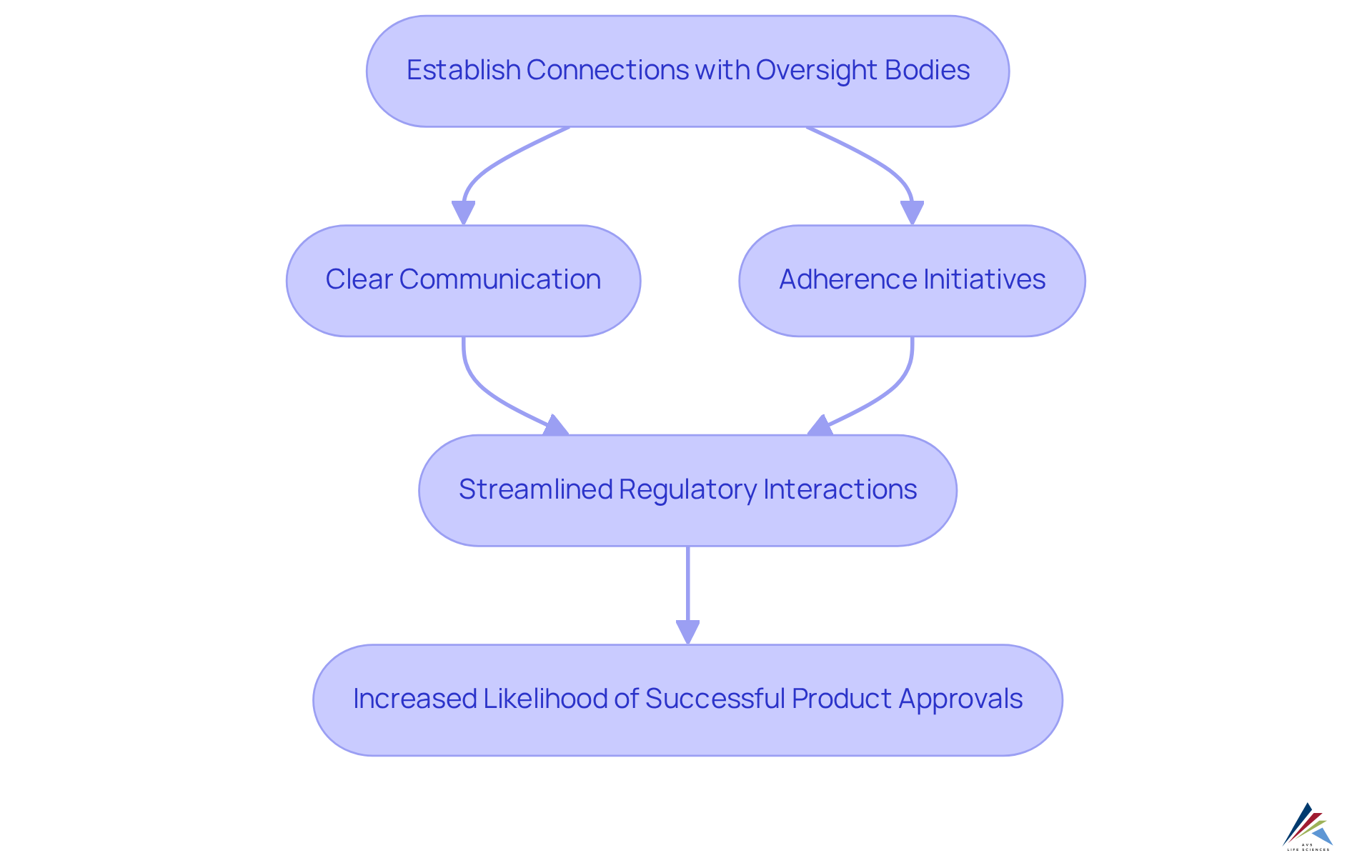
Regulatory Relationships: Building Trust with Compliance Authorities
Establishing robust connections with oversight bodies is essential for pharmaceutical firms navigating the complex compliance landscape. AVS Health Sciences serves as your dedicated partner in healthcare compliance consulting, ensuring thorough oversight, adherence, and quality solutions across biopharmaceuticals, medical devices, and nutraceuticals.
We assist clients in forging and maintaining these vital connections through . This trust not only streamlines regulatory interactions but also significantly increases the likelihood of successful product approvals.
Consequently, AVS Life Sciences emerges as a leader in healthcare compliance consulting as well as quality management solutions for the life sciences sector.
Conclusion
Healthcare compliance consulting stands as an indispensable asset for pharmaceutical companies striving to meet stringent regulatory standards while maintaining operational excellence. By leveraging expert guidance and tailored strategies, organizations can effectively navigate complex compliance landscapes, enhance risk management, and foster a culture of integrity and transparency.
This article highlights several key benefits of healthcare compliance consulting, including:
- Improved operational efficiency
- Effective risk management
- Comprehensive training programs
- Robust quality assurance frameworks
Each of these elements plays a critical role in safeguarding pharmaceutical operations, ensuring product safety, and maintaining high standards of quality. Furthermore, the proactive approach to compliance not only mitigates risks but also positions companies favorably in a competitive market, enabling them to focus on innovation and growth.
Ultimately, the significance of healthcare compliance consulting extends beyond mere adherence to regulations; it is about building trust with regulators, enhancing organizational resilience, and fostering a culture of ethical practices. As the pharmaceutical industry continues to evolve, investing in compliance consulting services will be essential for companies looking to thrive and ensure the safety and efficacy of their products in an increasingly complex regulatory environment.
Frequently Asked Questions
What services does AVS Life Sciences offer to pharmaceutical companies?
AVS Life Sciences offers a comprehensive suite of services including GMP audits, contract test laboratories, and data integrity services, all tailored to meet the distinct needs of pharmaceutical firms.
Why is healthcare compliance consulting important in the pharmaceutical industry?
Healthcare compliance consulting is crucial as it helps pharmaceutical companies navigate stringent regulations, enhances risk management, and ensures adherence to quality standards, thereby preventing severe penalties for non-compliance.
How does AVS Life Sciences support risk management for pharmaceutical operations?
AVS Life Sciences enhances risk management by identifying vulnerabilities through proactive risk evaluation methods, conducting thorough audits, and providing strategies to mitigate potential regulatory violations, ultimately safeguarding organizations against compliance penalties.
What are the financial implications of regulatory violations in the pharmaceutical sector?
A single regulatory misstep can lead to significant financial losses, with delays costing upwards of USD 500,000 in lost drug sales per day, in addition to possible penalties, highlighting the need for robust regulatory frameworks.
How can healthcare compliance consulting improve operational efficiency for pharmaceutical companies?
Healthcare compliance consulting helps streamline operations by identifying inefficiencies and implementing best practices, which saves time and reduces expenses associated with regulatory failures.
Can you provide an example of how AVS Life Sciences has improved a client's operations?
AVS Life Sciences assisted a leading biotechnology company in upgrading their manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility, completing the project on schedule and within budget while ensuring full traceability through meticulous documentation.
What technologies are being utilized to enhance compliance and productivity in the pharmaceutical sector?
Pharmaceutical companies are adopting Robotic Process Automation (RPA) and AI-driven automation tools, which have been shown to significantly improve adherence to regulations and increase productivity, with some reports indicating cost savings of up to 300% in the first year of implementation.
What is the overarching goal of AVS Life Sciences in the pharmaceutical industry?
The overarching goal of AVS Life Sciences is to empower pharmaceutical organizations to navigate regulatory complexities, foster a culture of compliance, and enhance operational efficiency, ultimately ensuring sustained success and patient safety.
