10 Benefits of Electronic Data Capture in Clinical Trials

Overview
The article delineates ten pivotal benefits of electronic data capture (EDC) in clinical trials, underscoring its critical role in elevating data quality, security, efficiency, and regulatory compliance. Evidence substantiates that EDC markedly diminishes errors linked to traditional methods, expedites study completion, and facilitates real-time data access. These advancements lead to improved operational efficiency and enhanced patient outcomes in clinical research.
Introduction
The landscape of clinical trials is undergoing a transformative shift as electronic data capture (EDC) systems emerge as pivotal tools for enhancing efficiency and accuracy. With the clinical research industry projected to grow significantly, the adoption of EDC solutions offers a myriad of benefits, from reducing human error to ensuring regulatory compliance. However, as organizations scramble to implement these advanced technologies, the question remains: how can they fully leverage EDC systems to optimize their clinical trial processes and outcomes? This article delves into ten key benefits of electronic data capture, revealing how these innovations are reshaping the future of clinical research.
1. AVS Life Sciences: Comprehensive Solutions for Electronic Data Capture in Clinical Trials
AVS Life Sciences offers expert support in selecting solutions designed to enhance electronic information capture (EDC) in clinical studies. By emphasizing regulatory compliance and quality management, the company effectively addresses the unique challenges encountered by pharmaceutical and biotechnology firms. Their extensive expertise in validation and engineering empowers clients to implement EDC systems that not only bolster information integrity but also streamline clinical processes.
As the demand for efficient information management escalates, the EDC market is projected to grow substantially. This growth is driven by the increasing complexity of clinical trials and the critical need for accurate information gathering.
Industry leaders emphasize that EDC solutions significantly reduce errors associated with traditional paper-based methods, ensuring information integrity and compliance with regulations such as HIPAA and GDPR. The integration of advanced technologies, including AI and machine learning, further enhances the capabilities of EDC frameworks, enabling predictive analytics and real-time information access.
With a strong commitment to regulatory adherence, AVS Life Sciences positions itself as a trusted partner in navigating the complexities of EDC implementation. This ensures that clients can leverage these tools to improve research efficiency and effectively meet regulatory requirements.
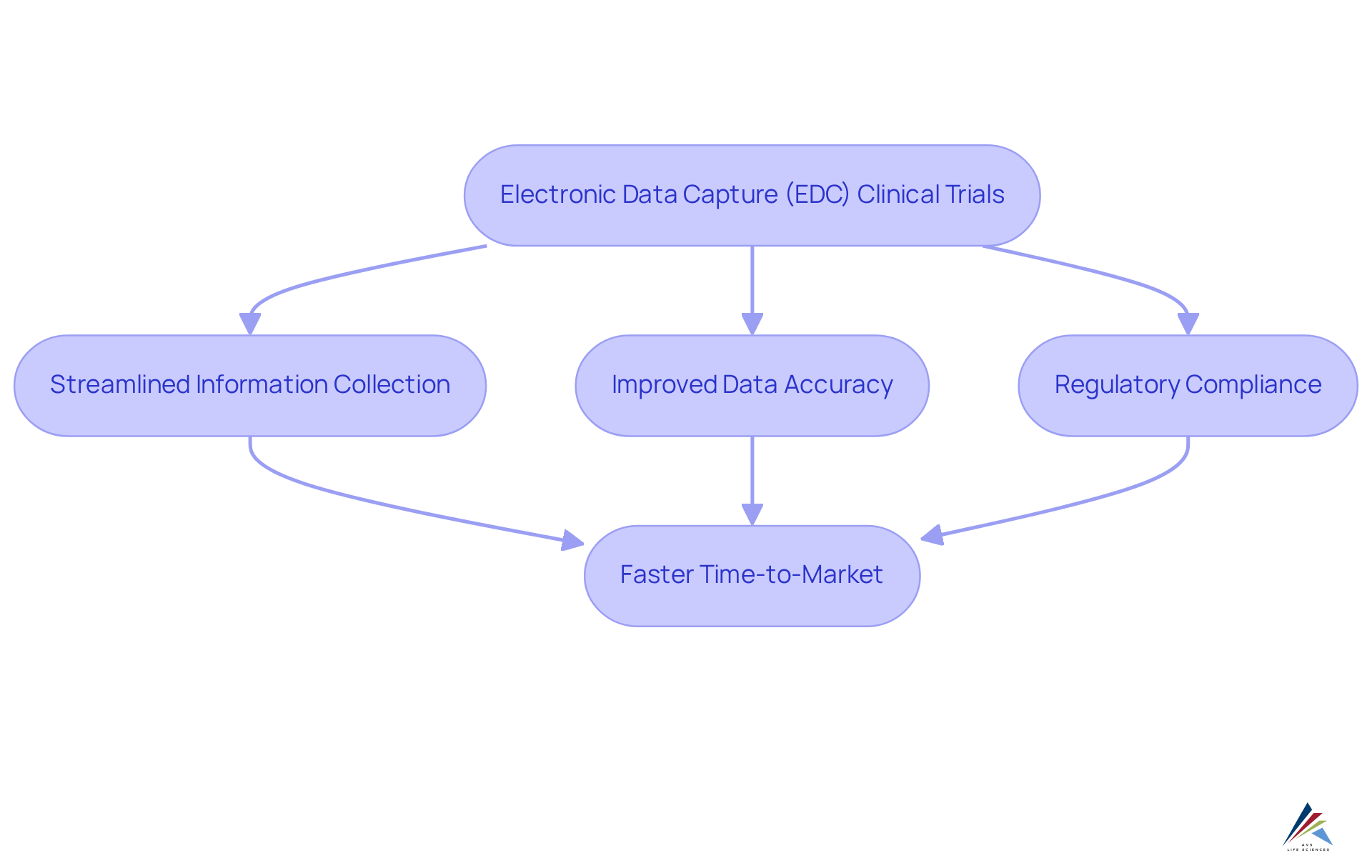
2. Streamlined Data Collection: Enhancing Efficiency in Clinical Trials
Electronic data capture clinical trials are revolutionized by electronic information gathering (EIG) platforms, which automate input and reduce reliance on traditional paper-based methods. This automation significantly mitigates human error. e by enhancing the speed of information collection, electronic data capture clinical trials empower clinical teams to focus on critical tasks while ensuring compliance with GXP and FDA regulations.
Such efficiency not only accelerates study completion but also optimizes resource allocation, facilitating faster market entry for new therapies.
Furthermore, EDC frameworks provide real-time access to information, enabling researchers to monitor patient progress and swiftly address any emerging issues, thereby circumventing costly delays and ensuring adherence to standard operating procedures (SOPs) and information integrity standards.
The integration of automated information entry processes has been shown to enhance experimental efficiency, with research indicating that experiments utilizing EDC can reduce operational costs compared to conventional methods. As the complexity of clinical studies continues to escalate, the adoption of electronic data capture in clinical trials becomes increasingly essential for ensuring compliance and enhancing overall research outcomes.
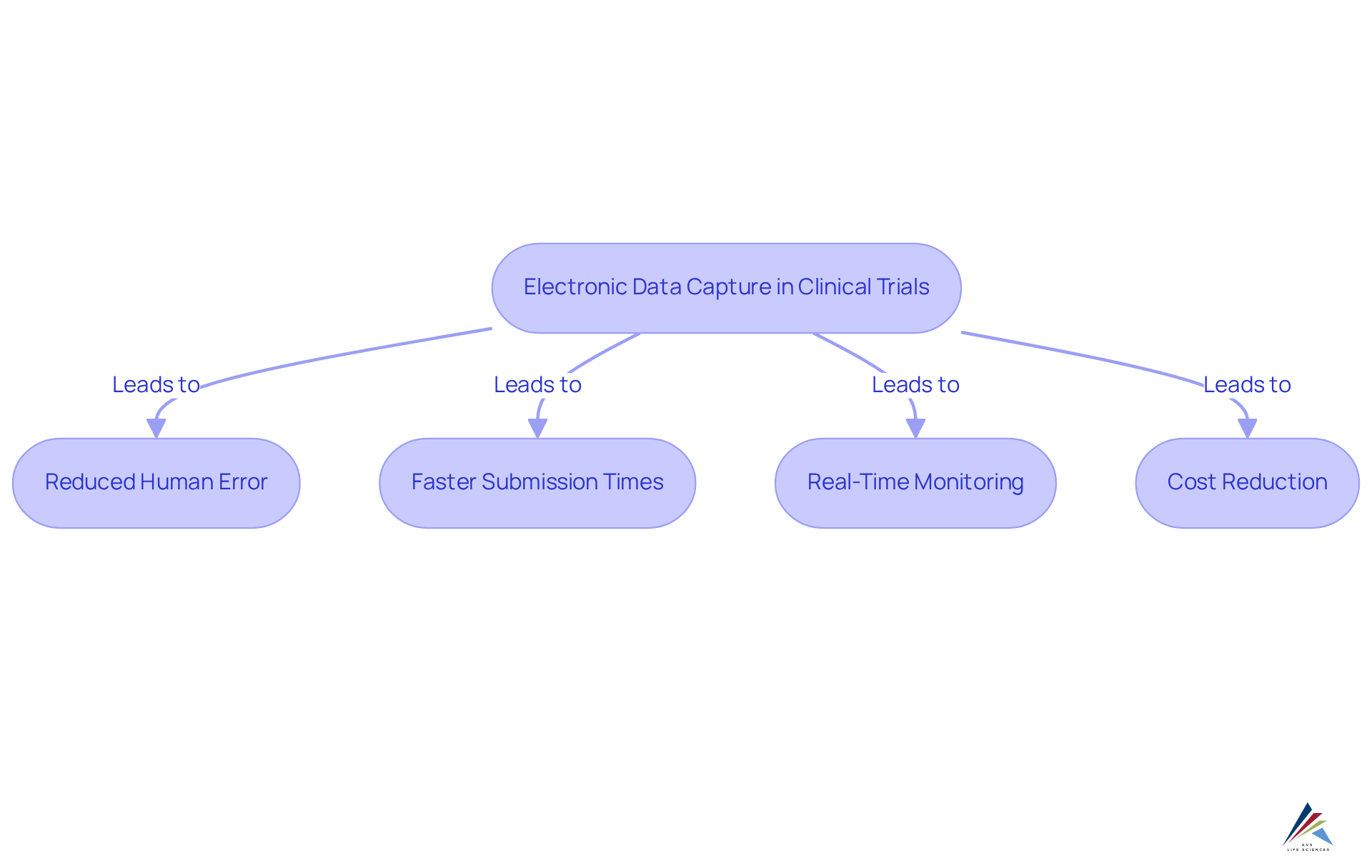
3. Improved Data Quality: Ensuring Accuracy in Clinical Research
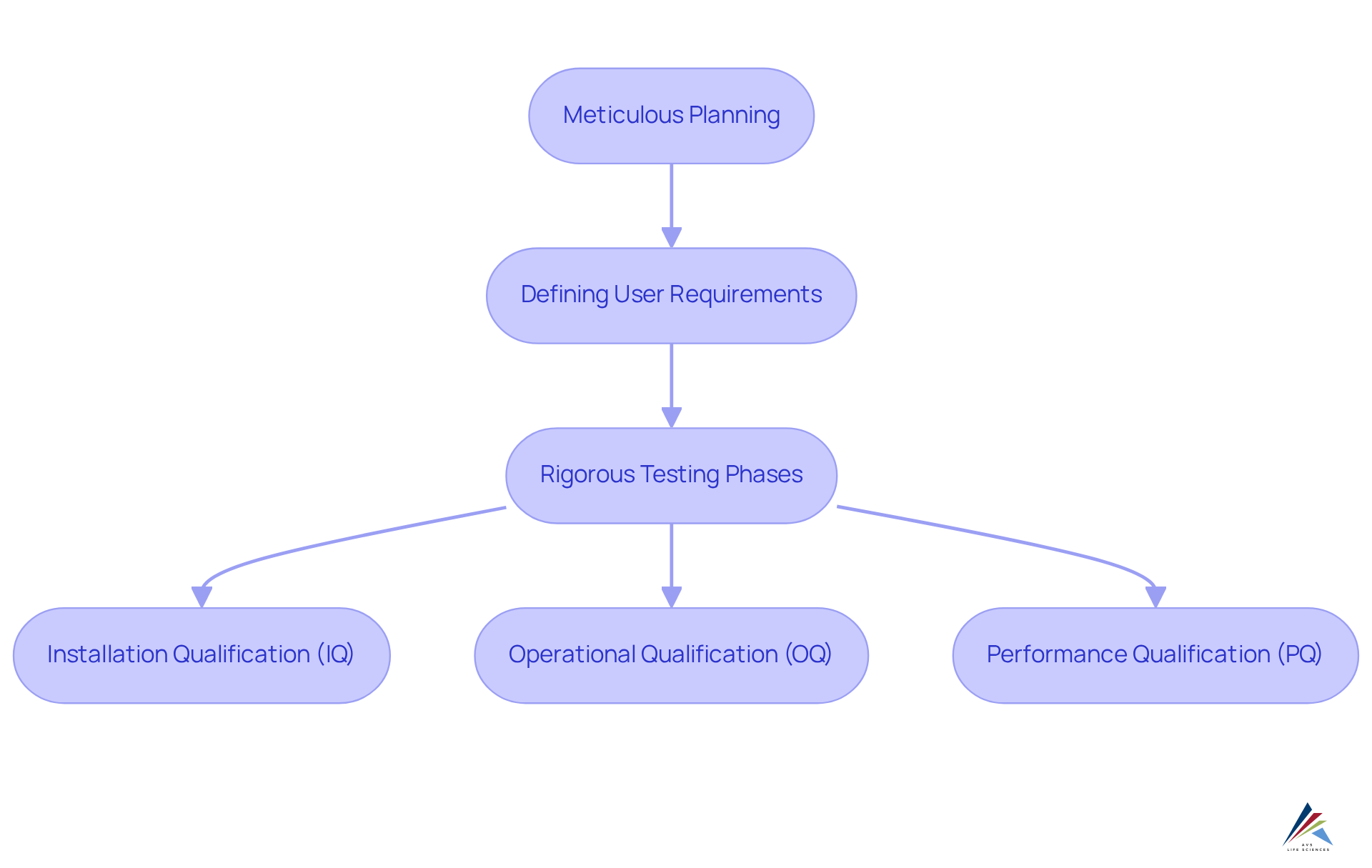
A significant advantage of electronic information collection (EDC) platforms lies in their ability to elevate the quality of information in clinical trials. These systems implement robust validation checks and automated data input processes, which substantially diminish the risk of human error. Central to this improvement is the computer application validation (CSV) process, as outlined in the Good Automated Manufacturing Practices (GAMP) 5 Guide. This process encompasses several stages, including:
- Meticulous planning
- Defining user requirements
- Rigorous testing phases such as:
- Installation Qualification (IQ)
- Operational Qualification (OQ)
- Performance Qualification (PQ)
By ensuring consistent and accurate information collection through integrated validation rules, EDC systems significantly enhance the reliability of clinical study outcomes, which is crucial for regulatory submissions and the protection of patient safety. It is imperative to recognize that precise information is not solely a regulatory requirement; it is a cornerstone of effective clinical research, directly influencing the validity of study outcomes and subsequent treatment decisions. To ensure compliance, it is vital for compliance officers to regularly evaluate the CSV process and its various stages, underscoring the importance of high-quality information in clinical studies.
4. Enhanced Data Security: Protecting Sensitive Clinical Trial Information
Data protection stands as a cornerstone in clinical studies, where sensitive patient information is paramount. Electronic data capture (EDC) platforms integrate advanced security features such as encryption, access controls, and comprehensive audit trails. These mechanisms are essential for thwarting unauthorized access and mitigating the risk of breaches, thereby safeguarding patient confidentiality throughout the trial process.
Encryption is pivotal in securing sensitive information within EDC systems. By rendering information unreadable to unauthorized users, encryption significantly reduces the potential ramifications of a security breach. Even in the event of compromised encrypted information, the consequences are markedly less severe than those stemming from breaches involving unencrypted data.
Access controls further bolster security by restricting information access to authorized personnel exclusively. Role-based access ensures that individuals can only view or manipulate information pertinent to their responsibilities, thereby minimizing the risk of unauthorized disclosures. Additionally, audit trails provide a detailed account of who accessed the information and when, fostering accountability and compliance with regulatory standards such as Good Clinical Practice (GCP) and HIPAA. Notably, over 90% of cyberattacks targeting the healthcare industry are phishing scams, underscoring the urgent necessity for robust security measures like audit trails to combat such threats.
As we look towards 2026, the landscape of clinical studies will evolve, making the integration of robust security measures in electronic data capture platforms indispensable. Organizations must prioritize these protections to uphold patient trust and adhere to stringent regulatory requirements. By implementing encryption and access controls, alongside comprehensive training for staff on security best practices, clinical studies can effectively safeguard sensitive information, ensuring the integrity and confidentiality of patient records throughout the research process.
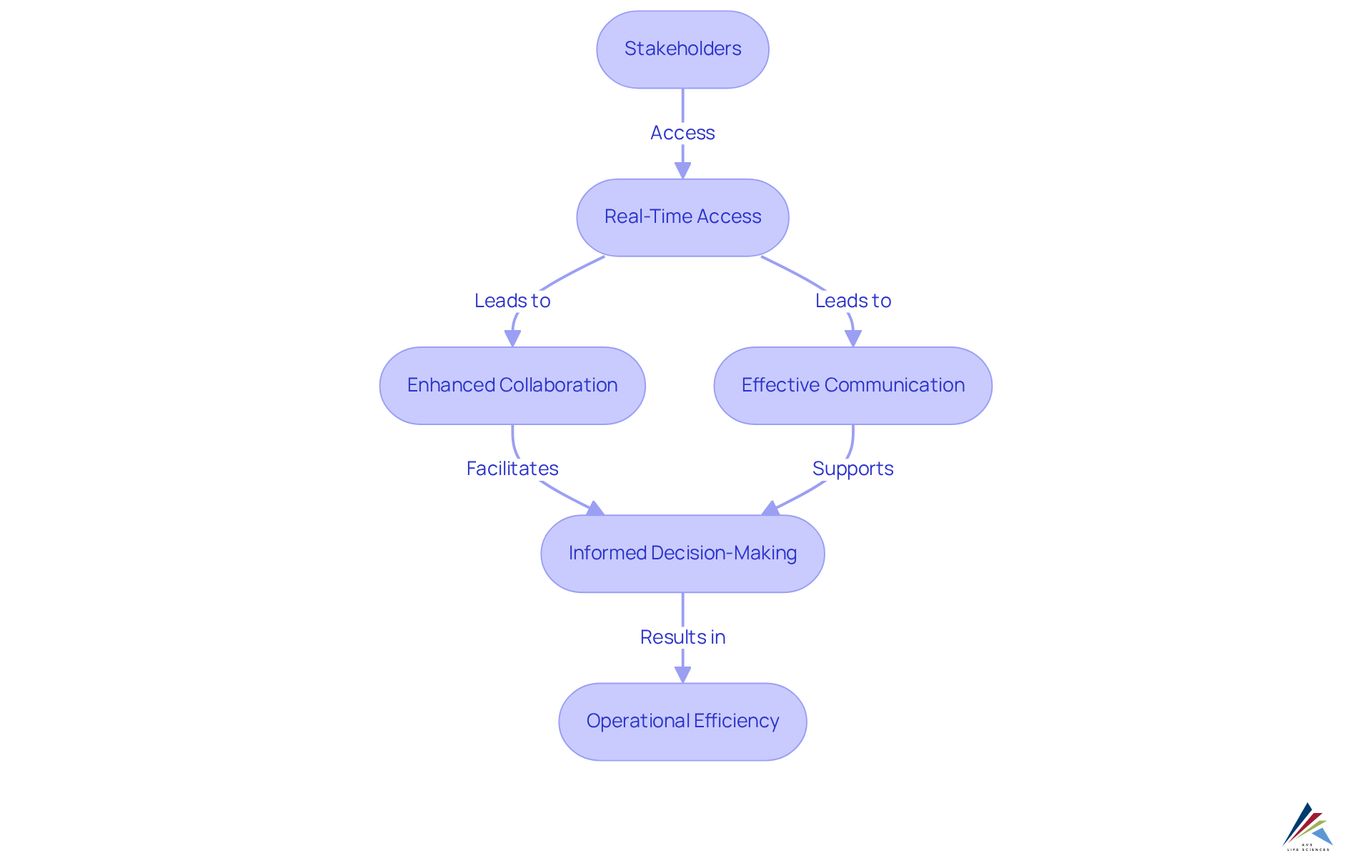
5. Increased Accessibility: Facilitating Collaboration in Clinical Trials
Electronic data collection (EDC) solutions significantly enhance accessibility by providing real-time access for diverse stakeholders, including clinical research groups, sponsors, and regulatory entities. This capability fosters collaboration, enabling more effective communication and informed decision-making throughout the clinical study process. By dismantling information barriers, EDC frameworks promote a unified management strategy, ultimately enhancing operational efficiency.
Industry experts underscore that effective communication among collaborators is paramount; streamlined processes can lead to quicker resolutions and improved study outcomes. As Dr. John Smith, a Clinical Study Specialist, notes, "The features of electronic information gathering frameworks in clinical studies not only improve information accuracy but also increase overall research efficiency, rendering them vital in modern clinical investigation."
As the clinical research landscape evolves, the integration of real-time information access remains a crucial factor in fostering successful partnerships and achieving shared objectives.
6. Accelerated Study Completion: Reducing Time-to-Market for Treatments
Electronic data capture in clinical trials significantly enhances the effectiveness of clinical studies by streamlining information collection and fostering collaboration among research groups. These technologies facilitate quicker data collection and immediate observation, enabling clinical teams to promptly identify and resolve issues, thereby minimizing delays in research progression. Such operational efficiency is paramount, as a majority of trials fail to meet enrollment timelines, resulting in costly setbacks.
Electronic data capture clinical trials can reduce operational costs, which directly contributes to a faster time-to-market for new therapies. For example, the implementation of EDC technologies has been shown to accelerate patient recruitment and retention, ensuring that qualified participants are enrolled more swiftly. Consequently, pharmaceutical companies can launch new therapies to the market more rapidly, ultimately benefiting patients in need of timely access to innovative treatments.
The integration of electronic data capture clinical trials technology not only enhances data accuracy but also supports compliance with regulatory standards, further smoothing the path from study initiation to market entry. AVS Life Sciences' quality management and regulatory compliance solutions can enhance electronic data capture in clinical trials, ensuring that clinical studies adhere to the highest standards of quality and efficiency.
As Hiren Thakkar asserts, 'As a leader, I am dedicated to empowering businesses to achieve their goals through innovative and cost-effective solutions.' This commitment to innovation is essential in addressing the challenges of managing multiple research sites, ensuring consistency and alignment throughout the entire clinical study process.
7. Cost Efficiencies: Maximizing Budget Utilization in Clinical Trials
Adopting electronic data capture solutions presents a compelling opportunity for significant cost savings in clinical studies. Drastically reducing reliance on paper-based processes and minimizing entry mistakes—where manual methods can compromise the records—EDC systems effectively lower operational expenses.
Automated EDC technologies achieve lower error rates in research settings, underscoring the advantages of automation over traditional methods. The automation of information gathering and analysis not only accelerates these processes but also empowers clinical teams to allocate resources more strategically, thereby maximizing budget utilization throughout the study.
For instance, organizations that have embraced electronic data capture in clinical trials report operational savings compared to traditional methods. This is primarily due to the substantial reductions in research costs facilitated by automating information gathering and minimizing manual tasks.
Furthermore, AVS Life Sciences' comprehensive quality management and regulatory compliance offerings enhance the efficiency of EDC platforms, ensuring that clinical studies adhere to industry standards. This transformation strengthens the overall integrity and efficiency of clinical research. Positioning electronic data captures clinical trials as essential tools for optimizing budgets in 2025 and beyond.
8. Real-Time Data Access: Enabling Immediate Insights in Clinical Trials
Immediate information access transforms clinical studies by empowering participants to oversee progress and make swift, informed decisions. Electronic data capture in clinical trials, offer immediate insights into study data, enabling clinical teams to quickly identify trends, address emerging challenges, and adjust protocols, as necessary. This capability not only enhances the overall efficiency of clinical research but also significantly influences decision-making processes.
As highlighted, immediate access to live information streams is increasingly vital for adherence and responsiveness in today's clinical study landscape. By integrating real-time visibility into clinical supervision, sponsors can proactively manage risks and ensure that operational processes align with regulatory expectations.
This underscores the significance of immediate information in regulatory contexts, facilitating proactive measures that conserve time, minimize risk, and safeguard the overall trajectory of the study.
9. Regulatory Compliance: Meeting Standards with Electronic Data Capture
Electronic information collection (EDC) frameworks are meticulously designed to adhere to rigorous regulatory standards, including Good Manufacturing Practices (GMP) and International Conference on Harmonisation (ICH) guidelines. By automating information collection procedures and maintaining comprehensive audit trails, electronic data capture clinical trials significantly enhance the integrity and precision of the information gathered during clinical trials. This automation simplifies information management and supports compliance with regulatory standards, thereby easing the process for inspections and approvals.
Compliance officers emphasize that EDC frameworks must produce secure, computer-generated, time-stamped audit trails that meticulously document user actions, including information entry, modifications, and deletions. Such features are crucial for preserving data integrity and ensuring that clinical studies meet the rigorous demands of regulatory scrutiny. For instance, the FDA’s 21 CFR Part 11 regulation mandates that electronic records and signatures used in clinical trials must be trustworthy and reliable, a requirement that EDC platforms be designed to fulfill.
Moreover, EDC frameworks have demonstrated their effectiveness in adhering to GMP and ICH guidelines through real-world applications. Organizations employing EDC platforms have reported improvement in information accuracy and reduction in testing times, underscoring the platforms' capacity to boost operational efficiency while ensuring compliance. This capability is especially vital in the context of decentralized clinical studies, where remote monitoring and data collection are becoming increasingly prevalent.
AVS Life Sciences offers comprehensive GXP regulatory services, including GMP audits for APIs, drug products, and testing facilities. The incorporation of electronic data capture in clinical trials platforms into clinical workflows not only aids adherence to these standards but also fosters a culture of quality and responsibility in clinical research, resulting in enhanced patient outcomes and expedited approval processes.
In summary, utilizing electronic data capture clinical trials solutions is crucial for compliance officers in the pharmaceutical sector, as these tools not only guarantee conformity to regulatory benchmarks but also enhance the overall effectiveness of clinical studies. The worldwide EDC market is projected to attain USD 4.20 billion by 2032, highlighting the increasing significance and investment in EDC solutions within the industry. Furthermore, the anticipated compound annual growth rate (CAGR) for EDC technologies is 10.60% throughout the forecast period of 2025 to 2032, emphasizing the growing importance of these technologies in the clinical research landscape.
10. Adaptability: Customizing Electronic Data Capture for Diverse Clinical Trials
A key benefit of electronic data capture in clinical trials solutions lies in their remarkable flexibility. These frameworks can be customized to meet the particular needs of different clinical studies, such as electronic data capture clinical trials, accommodating a broad spectrum of information types, research designs, and regulatory criteria. This intrinsic adaptability enables clinical teams to tailor their information gathering methods, ensure that their approaches for electronic data capture clinical trials align smoothly with the distinct goals of each study.
For instance, platforms such as Medidata's Rave EDC are designed to support intricate study designs and mid-study protocol modifications, thereby improving operational efficiency and information quality. Moreover, the incorporation of validated tools within electronic data capture in clinical trials frameworks facilitates standardized evaluations, enhancing data uniformity across various studies.
As highlighted by industry specialists, the capability to modify frameworks for electronic data capture clinical trials to the changing environment of clinical research is essential for enhancing study outcomes and ensuring adherence to regulatory standards. This flexibility not only optimizes workflows but also promotes collaboration among stakeholders, resulting in more successful electronic data capture clinical trials.
Additionally, using electronic data capture in clinical trials can reduce operational expenses, rendering them a financially attractive option for clinical studies.
As Medidata emphasizes, electronic data capture clinical trials require EDC platforms to have the flexibility to adapt to mid-study changes, highlighting the importance of this adaptability in ensuring compliance with global regulatory standards such as ICH-GCP, HIPAA, and 21 CFR Part 11. Conversely, the challenges posed by inflexible electronic data to capture clinical trials underscore the critical need for adaptability in clinical trials.
Conclusion
The integration of electronic data capture (EDC) in clinical trials signifies a transformative shift towards enhanced efficiency, accuracy, and compliance in research processes. By automating data collection and minimizing reliance on traditional methods, EDC solutions not only streamline workflows but also significantly reduce the potential for human error, thereby ensuring that clinical studies adhere to rigorous regulatory standards.
Key benefits of EDC are evident, including:
- Improved data quality through robust validation checks
- Enhanced data security with advanced encryption and access controls
- Increased accessibility that fosters collaboration among stakeholders
Moreover, the ability to access real-time data empowers clinical teams to make informed decisions swiftly, accelerating study completion and reducing time-to-market for new therapies. These advantages underscore the critical role of EDC in modern clinical research.
As the clinical trial landscape continues to evolve, the adoption of electronic data capture systems becomes essential for organizations aiming to optimize their research processes and maintain regulatory compliance. Embracing these innovative solutions is not merely a trend; it is a necessary strategy to enhance the quality of clinical research, ensure patient safety, and facilitate the timely delivery of new treatments to those in need. The future of clinical trials hinges on the effective utilization of EDC technologies, making it imperative for industry stakeholders to prioritize their implementation and integration.
Frequently Asked Questions
What solutions does AVS Life Sciences offer for electronic data capture in clinical trials?
AVS Life Sciences provides a comprehensive solutions designed to enhance electronic information capture (EDC) in clinical studies, focusing on regulatory compliance and quality management to address challenges faced by pharmaceutical and biotechnology firms.
How do EDC solutions improve data integrity compared to traditional methods?
EDC solutions significantly reduce errors associated with traditional paper-based methods, ensuring information integrity and compliance with regulations such as HIPAA and GDPR, while integrating advanced technologies like AI and machine learning for enhanced capabilities.
What validation processes do EDC platforms implement to ensure data quality?
EDC platforms utilize robust validation checks and automated data input processes, including the computer application validation (CSV) process, which involves meticulous planning, defining user requirements, and rigorous testing phases such as Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ).
Why is accurate information crucial in clinical research?
Precise information is essential not only for regulatory compliance but also for the validity of study outcomes and subsequent treatment decisions, influencing patient safety and the reliability of clinical study results.
