10 Benefits of Being GMP Compliant for Pharmaceutical Companies

Overview
The article delineates ten pivotal benefits of GMP compliance for pharmaceutical companies, highlighting:
- Enhanced product safety
- Regulatory adherence
- Operational efficiency
- Improved product quality
- Heightened consumer confidence
These benefits are substantiated by case studies and industry insights that illustrate how GMP compliance not only mitigates risks and curtails costs but also fosters innovation and fortifies market position. This strategic positioning equips organizations for enduring success within a competitive landscape.
Introduction
Pharmaceutical companies navigate a landscape characterized by stringent regulations and an unwavering demand for product safety and quality. By embracing Good Manufacturing Practices (GMP) compliance, organizations not only adhere to these regulations but also unlock a multitude of benefits that can significantly enhance operational efficiency and product integrity. Yet, the challenge persists: how can organizations effectively navigate the complexities of GMP compliance to leverage these advantages while maintaining a competitive edge? This article delves into the ten key benefits of being GMP compliant, illustrating how pharmaceutical firms can transform compliance from a mere obligation into a strategic asset.
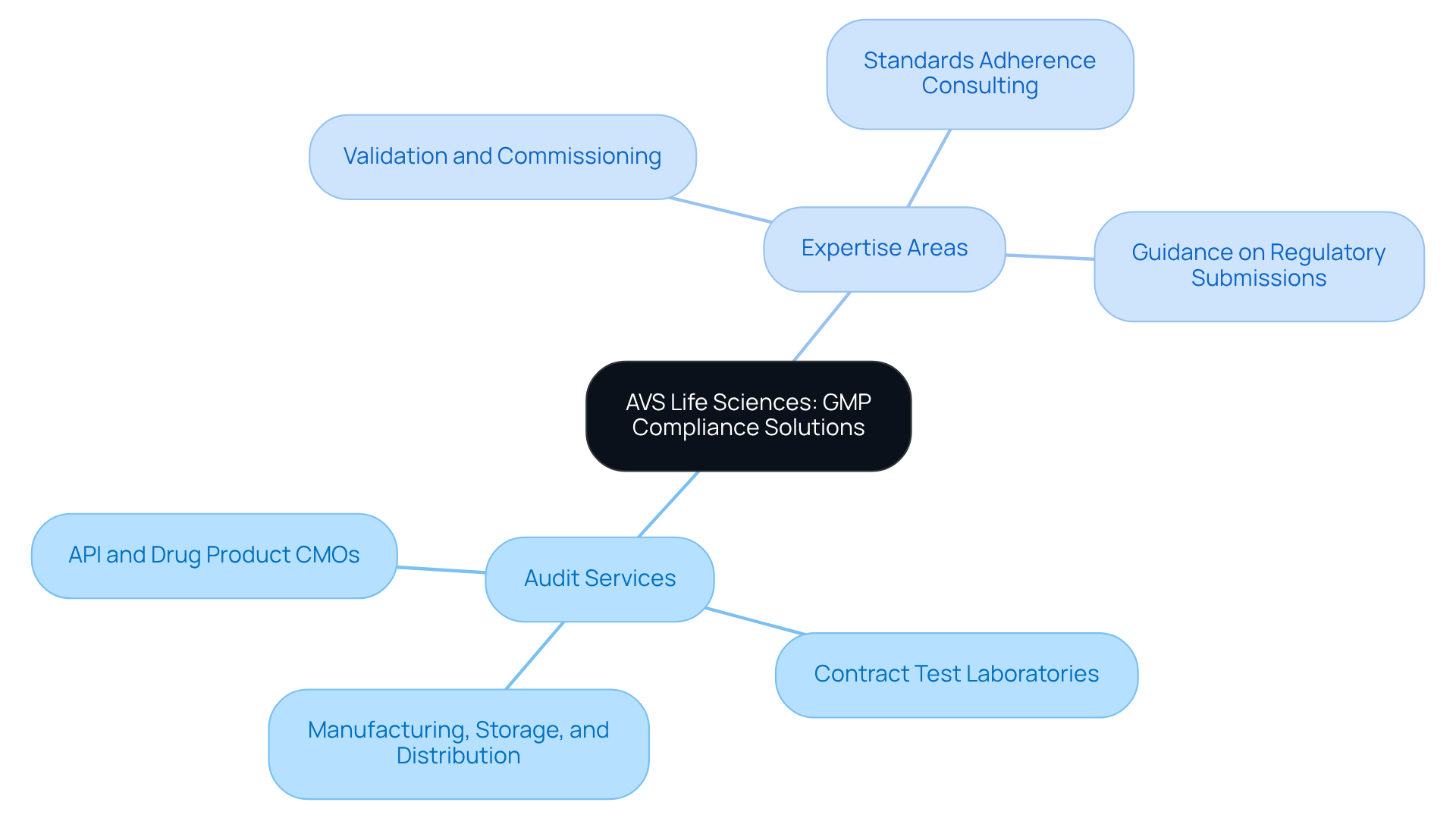
AVS Life Sciences: Comprehensive GMP Compliance Solutions for Quality Assurance
stands at the forefront of , meticulously tailored to meet the unique demands of pharmaceutical firms. The company's offerings include comprehensive audits that are GMP compliant for:
- API and drug product CMOs
- Contract test laboratories
- Manufacturing, storage, and distribution locations
This ensures compliance across all critical areas. Additionally, their expertise extends to:
- Standards adherence consulting
With a robust team of over 300 seasoned professionals, the complexities of regulatory environments, upholding exceptional quality standards throughout the product lifecycle. Their approach to and bespoke strategies designed to ensure that they remain GMP compliant while also enhancing operational efficiency. By partnering with AVS Life Sciences, organizations can confidently address compliance challenges and achieve sustainable success in their operations.
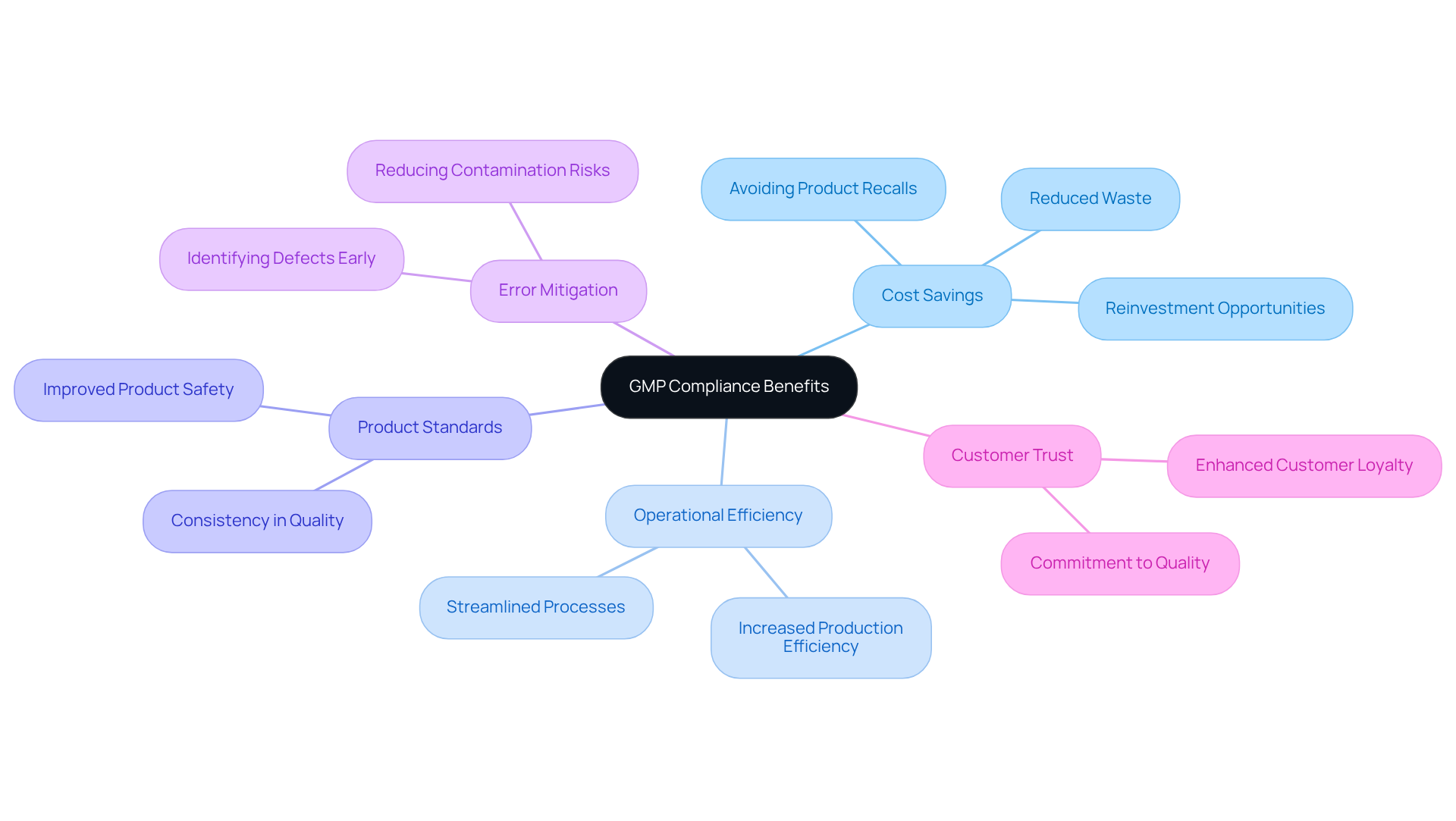
Enhanced Product Safety: Assurance Through GMP Compliance
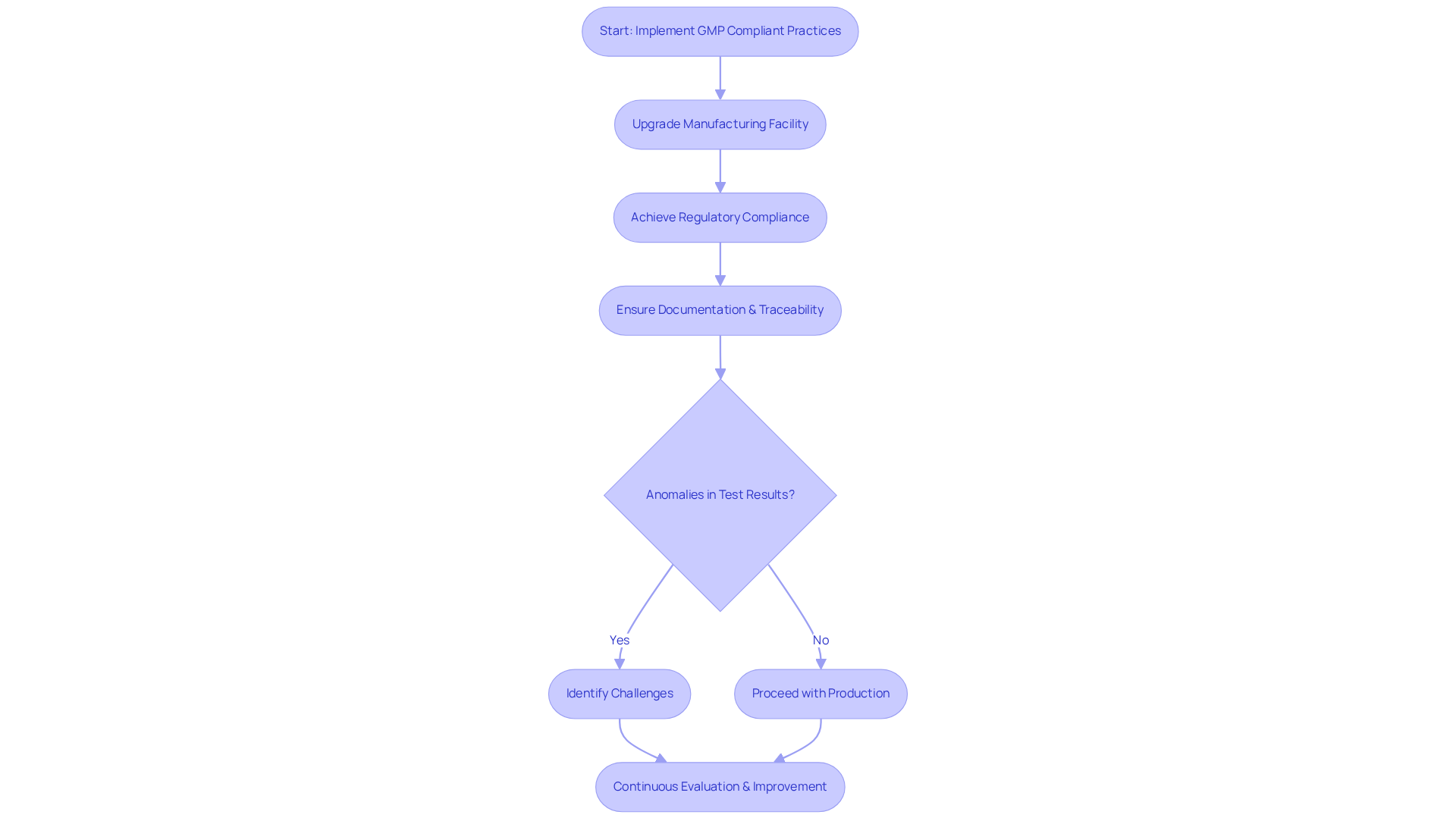
practices are crucial for enhancing product safety through the implementation of stringent manufacturing processes that significantly mitigate the risk of contamination and defects. By ensuring their practices are GMP compliant, that their products consistently meet safety standards, thereby reducing the likelihood of recalls and adverse health effects.
A compelling case study from . The company effectively aided a leading biotechnology firm in upgrading its manufacturing facility from a Biosafety Level 1 GMP environment to a Level 2 GMP facility. This upgrade not only achieved but also emphasized the throughout the manufacturing process.
AVS Life Sciences' provided complete traceability, as verified by the client’s assurance team. Throughout this endeavor, challenges, such as anomalies in test results, were identified, leading to valuable insights that prompted the client to reassess their business processes.
Such thorough are vital for ensuring that pharmaceutical products are GMP compliant and adhere to the highest safety standards, ultimately benefiting patients and bolstering the overall integrity of the life sciences sector. To further capitalize on the advantages of , pharmaceutical firms must routinely evaluate their procedures and implement improvements based on the insights gained.
Regulatory Compliance: Navigating Complex Standards with GMP
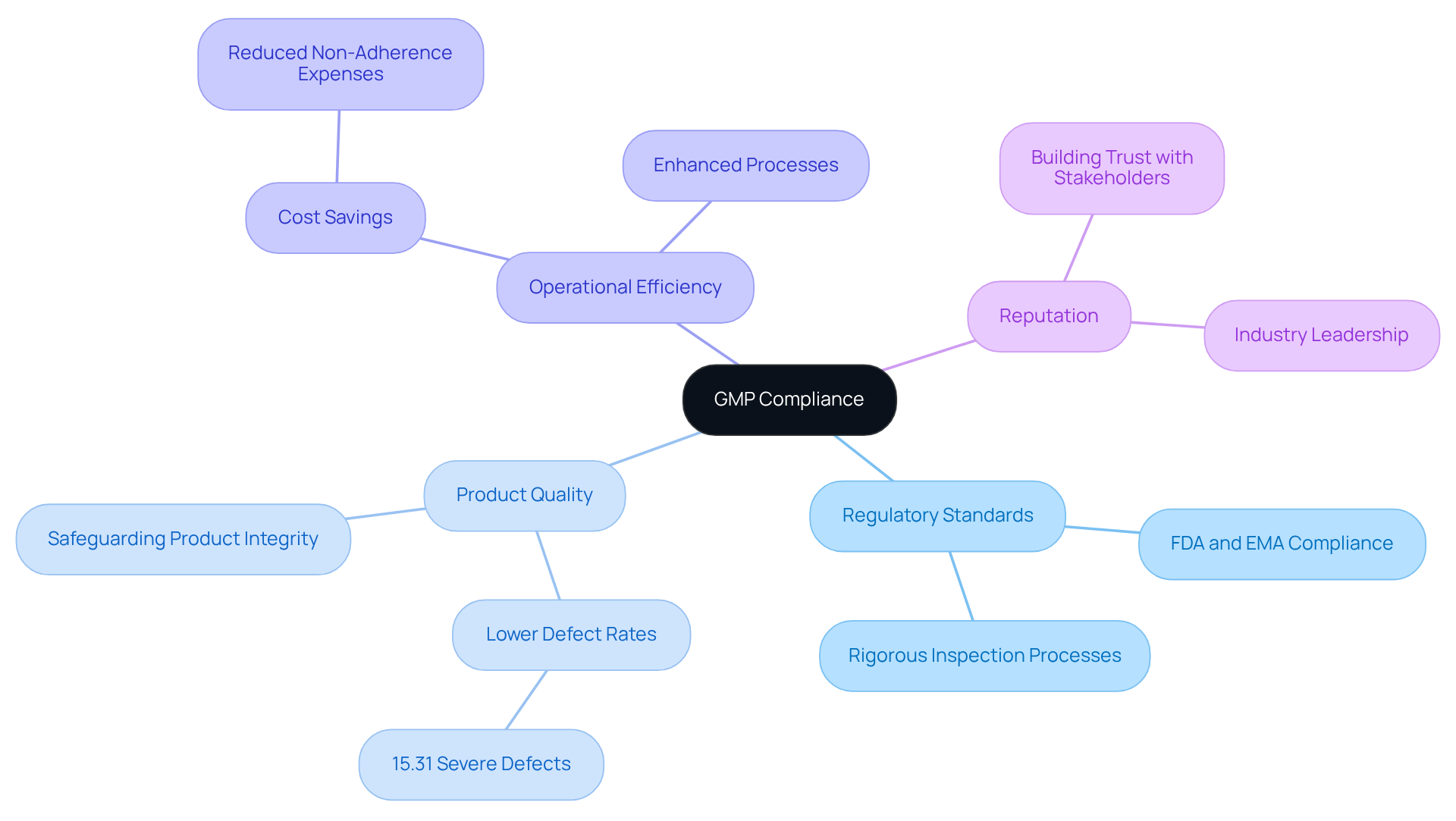
serves as a critical framework for navigating the complex regulatory standards established by agencies such as the FDA and EMA. By adopting , pharmaceutical firms can effectively streamline their compliance processes, ensuring adherence to all necessary regulations while maintaining high-quality standards. This proactive approach significantly reduces the risk of regulatory penalties. Studies show that firms with encounter lower rates of severe defects during inspections—only 15.31% of organizations surveyed reported severe defects in GMP inspections.
Moreover, the implementation of product quality but also enhances a company's reputation within the industry. Industry leaders stress that is crucial for building trust among stakeholders and consumers. Organizations that emphasize [GMP adherence](https://nelsonlabs.com/news/european-union-grants-a-good-manufacturing-practice-certificate-to-nelson-laboratories) often report enhanced operational efficiency and lowered expenses related to non-adherence.
The effect of GMP on adherence rates is clear. Organizations that invest in tend to achieve improved results in . The FDA's rigorous inspection processes, based on standard approaches and reports of potentially defective drug products, highlight the importance of being with guidelines. Furthermore, the typical count of standard operating procedures set by businesses is 578.69, indicating the extensive actions organizations undertake to ensure adherence.
In summary, navigating through GMP compliant practices aids adherence and positions pharmaceutical firms for long-term success in a highly regulated environment. By prioritizing GMP, organizations can enhance their operational integrity and maintain a competitive edge in the market. For additional insights on GMP adherence, please refer to our FAQs section, which addresses common questions and concerns related to regulatory practices.
Operational Efficiency: Streamlining Processes with GMP Practices
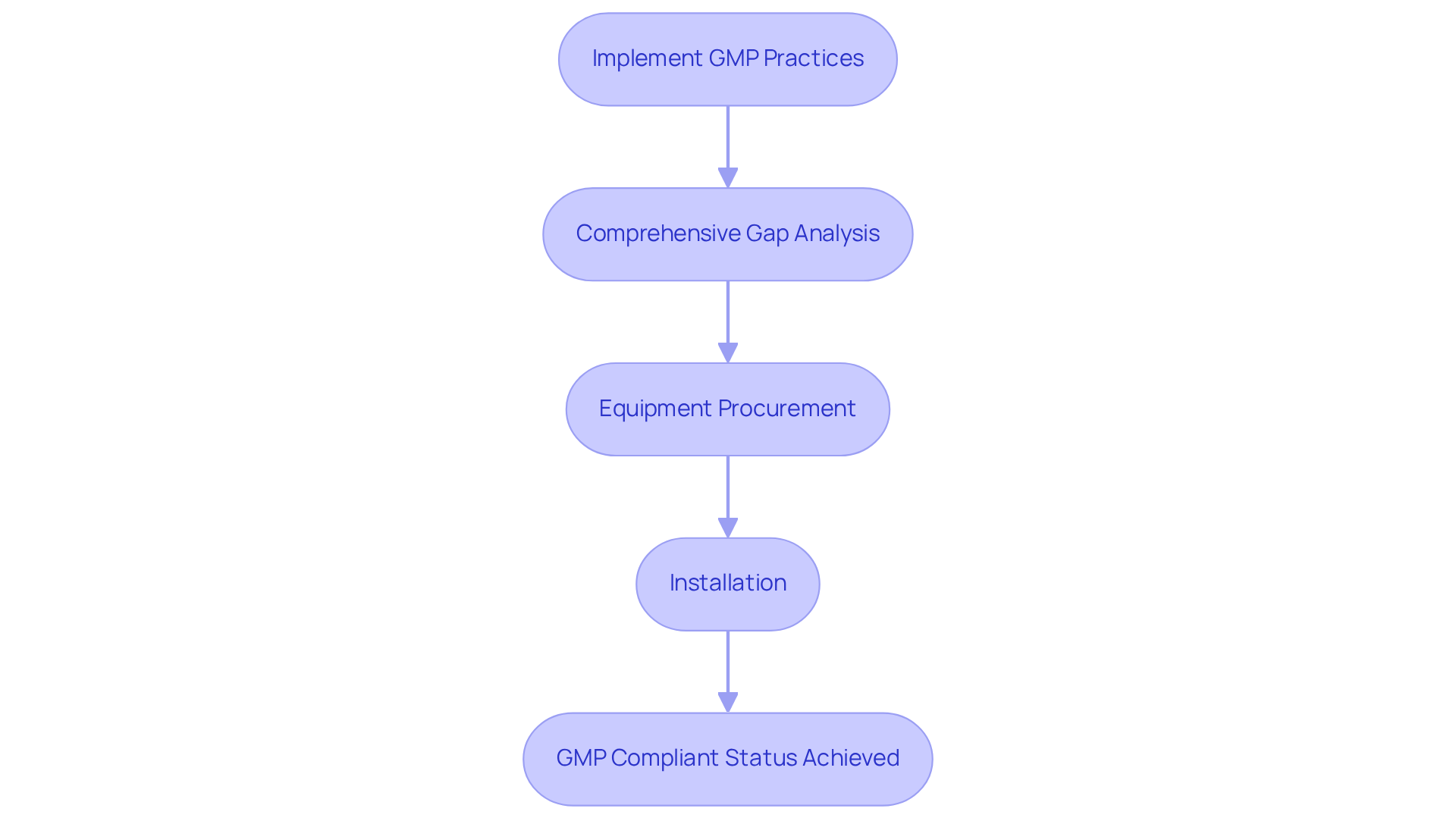
Implementing significantly enhances and minimizing variability. This streamlining of operations not only , allowing pharmaceutical firms to produce more effectively.
Organizations that adopt GMP practices often report a reduction in production costs by as much as 20%, attributed to improved resource management and waste minimization. A notable case study illustrates how in upgrading their facility from a Biosafety Level 1 to a Level 2 environment that is for lentivirus production. This transformation involved:
- Equipment procurement
- Installation
All completed within a tight 9-month timeline. Such successful upgrades demonstrate that companies can and maintaining .
By concentrating on process standardization and careful documentation, these organizations can attain consistent product standards, ultimately fostering greater trust among consumers and regulatory bodies alike.
Improved Product Quality: The Direct Impact of GMP Standards
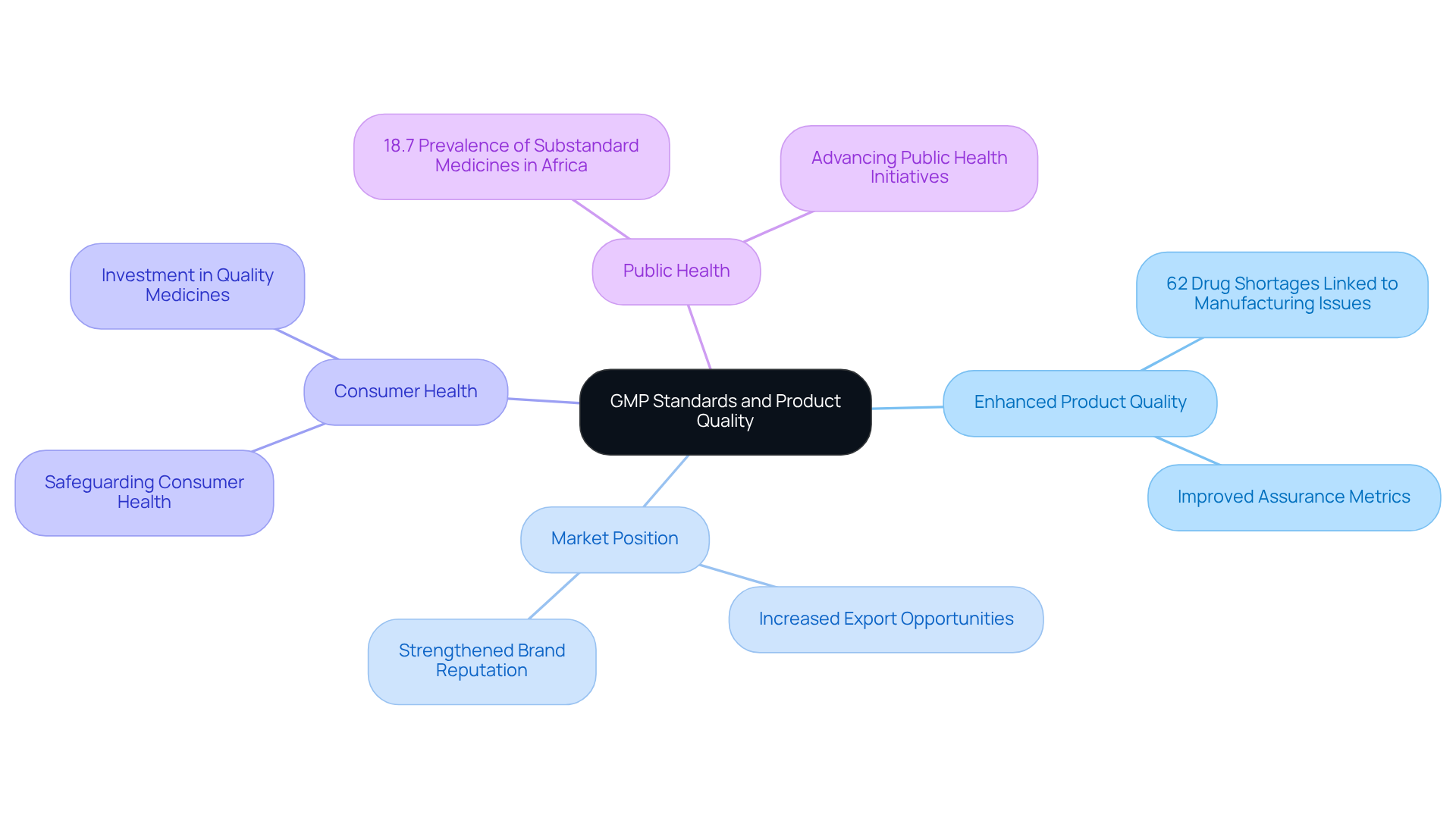
standards are essential for ensuring that pharmaceutical products are . Adherence to these standards is directly linked to , as evidenced by a global study indicating that organizations with advanced management practices experience significantly fewer issues and improved efficiency. Notably, in drug shortages, with 62% of shortages from 2013 to 2017 attributed to manufacturing or product-related problems.
Moreover, compliance with GMP not only safeguards consumer health but also strengthens an organization's market position and brand reputation. Pharmaceutical companies that meet through GMP practices often witness , leading to increased export opportunities. In Africa, for instance, enhancing GMP compliance is vital for tackling the 18.7% prevalence of , thereby advancing public health and revitalizing the local pharmaceutical sector.
Recent assurance reports from firms that are GMP compliant illustrate that the effective implementation of these standards results in enhanced delivery performance and reliability in drug supply. This is further corroborated by client testimonials that underscore the success of AVS Life Sciences in managing , ensuring that clients reach their quality objectives. Ultimately, a not only protects consumers but also positions organizations as leaders in the competitive pharmaceutical landscape.
Competitive Advantage: Standing Out with GMP Compliance
Achieving is a critical differentiator for pharmaceutical firms competing in the landscape. By demonstrating a steadfast commitment to quality and safety, businesses can not only attract a broader client base but also forge stronger connections with stakeholders. GMP certification serves as a hallmark of excellence, signaling to clients and partners that the organization prioritizes high operational standards.
and expert guidance to help pharmaceutical firms navigate the complexities of staying GMP compliant. Our approach ensures that companies meet regulatory obligations while simultaneously enhancing their operational efficiency. With our proven track record in successful compliance projects, we illustrate our expertise and commitment to excellence in the field.
In today's regulatory environment, the stakes are high. Non-compliance can lead to significant repercussions, including financial penalties and reputational damage. Therefore, is not merely a choice but a for firms aiming to excel. Let us assist you in becoming GMP compliant, reinforcing your commitment to quality, and positioning your organization as a leader in the industry.
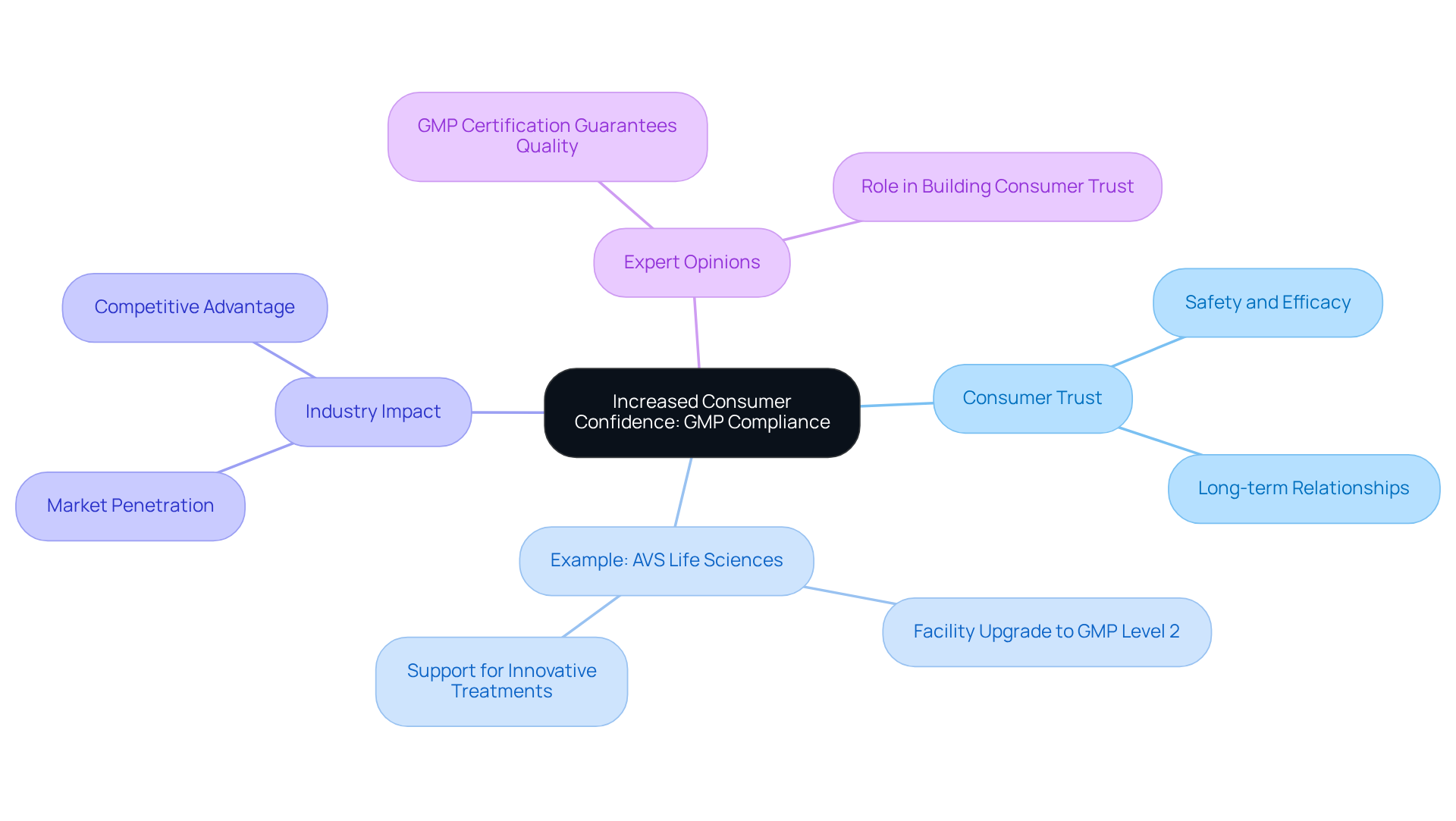
Increased Consumer Confidence: Trust Built on GMP Compliance
Adhering to practices significantly enhances by ensuring that pharmaceutical products are manufactured under rigorous standards. When consumers recognize a product as GMP compliant, their confidence in its safety and efficacy increases markedly. This elevated trust is essential for nurturing long-term customer relationships and fostering brand loyalty.
For instance, a leading biotechnology company in upgrading their from a Biosafety Level 1 environment to a . This successful transition not only but also allowed the client to focus on developing innovative treatments for serious illnesses.
Such collaborations demonstrate that being not only assures consumers of product excellence but also strategically positions brands in a competitive landscape, ultimately cultivating loyalty and trust in their offerings.
As industry specialists emphasize, 'GMP compliant and regulated according to standards of excellence,' underscoring the critical role of adherence in building consumer trust.
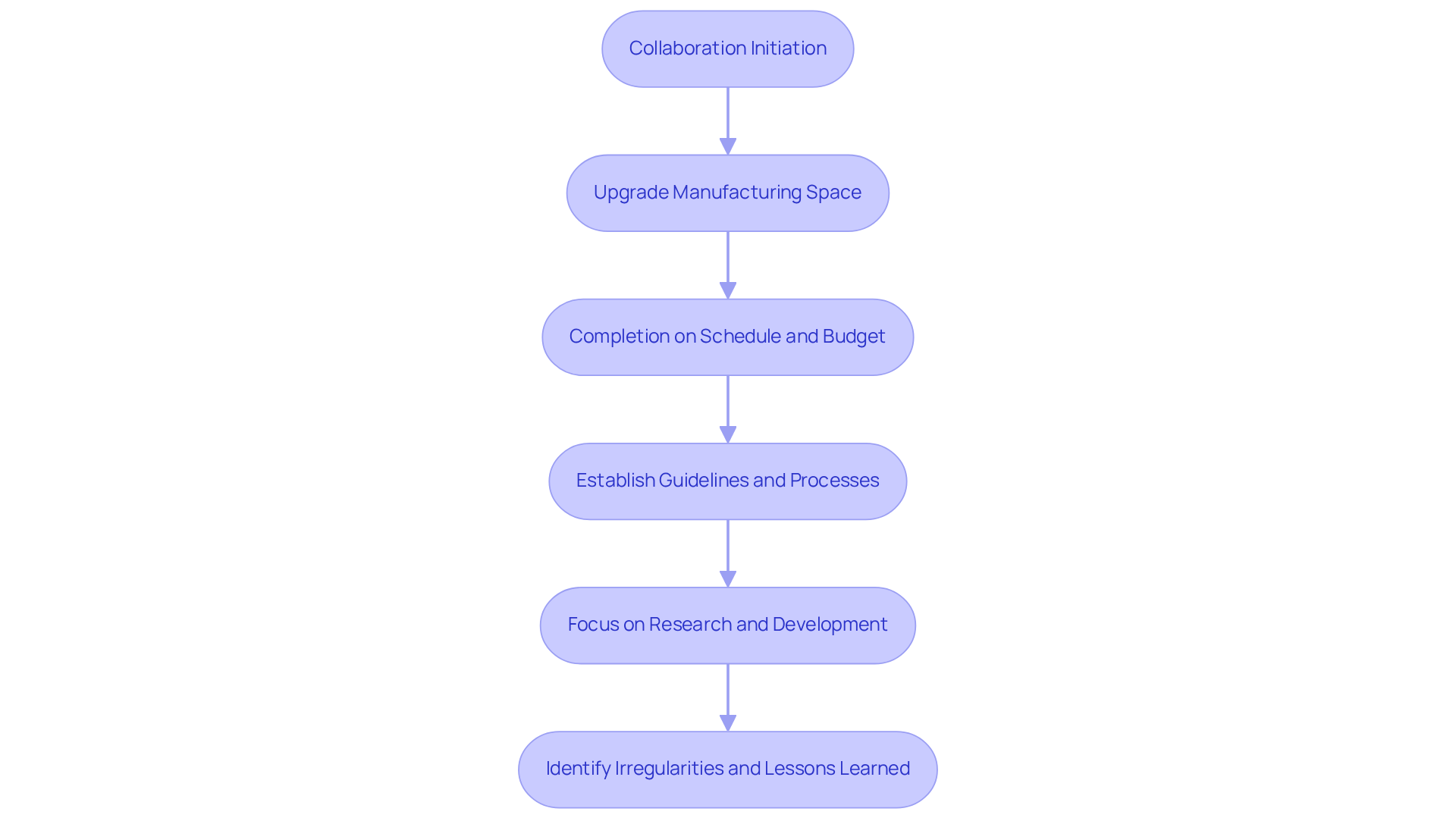
Fostering Innovation: Enabling Development Through GMP Standards
not only guarantee product excellence but also foster an environment conducive to innovation. A prime example of this is with a leading biotechnology firm, where we facilitated the upgrade of their manufacturing space from a to a Level 2 GMP facility for lentivirus production. This enhancement was completed on schedule and within budget, demonstrating how can drive operational advancements without sacrificing quality.
By establishing clear guidelines and processes, including , organizations can focus on research and development, exploring novel ideas and technologies. Our comprehensive approach, which encompassed , enabled our client to concentrate on their core mission: developing with serious diseases.
Throughout the project, we identified irregularities in test results stemming from improperly installed barcode scanner cameras, yielding significant lessons that . This balance ultimately culminates in the development of .
Cost Savings: Long-Term Financial Benefits of GMP Compliance
Investing in may necessitate initial expenditures; however, the long-term are considerable. Companies that are GMP compliant can expect significant and reduced waste. A prime example is , which assisted a leading biotechnology firm in elevating their manufacturing area from a Biosafety Level 1 GMP site to a Level 2 GMP site. This transformation illustrates how such enhancements can lead to and superior product standards.
The collaboration not only curtailed expenses but also allowed the client to focus on developing medicines that improve patient well-being. Other organizations can achieve similar results through , as it mitigates costly errors and boosts production efficiency. The financial repercussions of product recalls for firms that are not GMP compliant can be devastating, often resulting in losses that far exceed the initial investments in compliance strategies.
Furthermore, organizations that successfully adopt GMP practices can reinvest these savings into further improvements, creating a cycle of continuous enhancement. As noted by National Vitamin Company, 'As leaders in the industry, we are proud of our dedication to following good manufacturing practices with any job outsourced to us.' This commitment to being not only cultivates customer trust but also leads to long-term financial stability and growth.
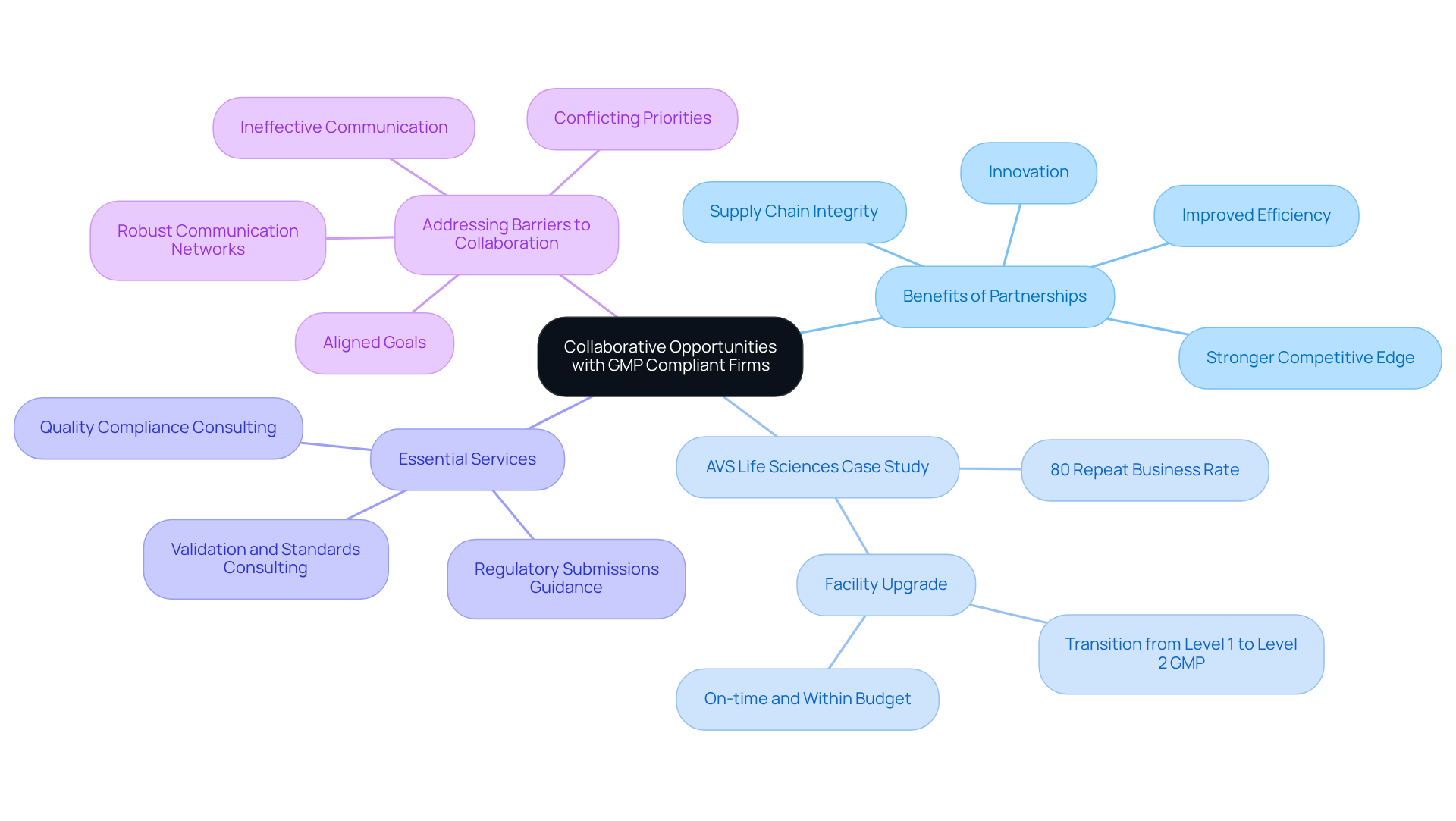
Collaborative Opportunities: Building Partnerships with GMP Compliant Firms
among pharmaceutical firms and their stakeholders, including suppliers and distributors. By forming alliances with , companies can significantly enhance their supply chain integrity, ensuring that every production aspect adheres to stringent standards. A prime example is AVS Life Sciences, which successfully upgraded a biotechnology GMP facility by assisting a leading San Francisco-based biotechnology company in its transition from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. This project was completed on time and within budget, demonstrating AVS's .
such as validation and standards consulting, which are critical for bolstering these partnerships and enhancing overall supply chain integrity. These collaborations not only and broaden market reach. Companies engaged in such cooperative efforts frequently report improved efficiency and a stronger competitive edge, leveraging shared expertise and resources. Notably, AVS Life Sciences boasts an impressive , highlighting the effectiveness of its partnerships in driving success.
The dedication to quality and GMP compliant standards within these collaborations is vital, as it , ultimately benefiting all involved parties. Furthermore, AVS Life Sciences proactively addresses common barriers to collaboration, such as ineffective communication and conflicting priorities, by establishing robust communication networks and promoting aligned goals.
Conclusion
GMP compliance stands as a cornerstone for pharmaceutical companies striving for excellence in product safety, regulatory adherence, and operational efficiency. By integrating Good Manufacturing Practices into their operations, organizations not only enhance product quality but also establish a robust foundation for sustainable success in a highly competitive landscape.
The exploration of GMP compliance reveals key benefits, such as:
- Improved product safety through stringent manufacturing processes
- Streamlined navigation of complex regulatory standards
- Significant operational efficiencies that lead to cost savings
Additionally, the establishment of consumer trust and the fostering of innovation underscore the multifaceted advantages of adhering to GMP standards, positioning firms as leaders in the pharmaceutical sector.
In an industry where quality and safety are paramount, the commitment to GMP compliance transcends regulatory necessity; it emerges as a strategic advantage. Pharmaceutical companies are urged to embrace these practices, not merely to meet compliance requirements but to cultivate a culture of excellence that drives innovation and enhances market position. By prioritizing GMP compliance, organizations can secure their reputation, build lasting consumer confidence, and ultimately contribute to the advancement of public health.
Frequently Asked Questions
What does AVS Life Sciences offer for GMP compliance?
AVS Life Sciences provides comprehensive GMP compliant solutions, including audits for API and drug product CMOs, contract test laboratories, and manufacturing, storage, and distribution locations. They also offer validation and commissioning, standards adherence consulting, and guidance on regulatory submissions.
How does GMP compliance enhance product safety?
GMP compliant practices implement stringent manufacturing processes that reduce the risk of contamination and defects, ensuring products consistently meet safety standards. This helps to minimize the likelihood of recalls and adverse health effects.
Can you provide an example of GMP compliance in action?
AVS Life Sciences assisted a biotechnology firm in upgrading its manufacturing facility from a Biosafety Level 1 GMP environment to a Level 2 GMP facility. This upgrade achieved regulatory compliance and highlighted the importance of maintaining GMP compliance throughout the manufacturing process.
What role does GMP compliance play in regulatory standards?
GMP adherence serves as a critical framework for navigating complex regulatory standards set by agencies like the FDA and EMA. It streamlines compliance processes, ensuring adherence to regulations while maintaining high-quality standards and reducing the risk of regulatory penalties.
How does GMP compliance affect a company's reputation?
Being GMP compliant enhances a company's reputation by building trust among stakeholders and consumers. Organizations that prioritize GMP adherence often experience improved operational efficiency and reduced expenses related to non-compliance.
What are the benefits of investing in quality management systems related to GMP?
Investing in quality management systems leads to improved results in regulatory inspections and lower rates of severe defects during inspections. Organizations with strong GMP systems report better adherence rates and overall operational integrity.
How many standard operating procedures do organizations typically implement for GMP compliance?
The typical count of standard operating procedures set by businesses to ensure GMP adherence is approximately 578.69, indicating the extensive measures organizations take to comply with regulations.
Where can I find more information about GMP adherence and regulatory practices?
For additional insights on GMP adherence, you can refer to the FAQs section that addresses common questions and concerns related to regulatory practices.
