10 Essential GMP Practices for Pharmaceutical Compliance Officers

Overview
The article underscores the crucial Good Manufacturing Practices (GMP) that pharmaceutical compliance officers must embrace to ensure regulatory adherence and uphold product quality. It highlights the significance of:
- Effective documentation
- Comprehensive training
- Rigorous internal audits
- Expert consulting services
These practices are asserted to substantially diminish compliance errors and boost operational efficiency, thereby cultivating a culture of continuous improvement within the pharmaceutical sector.
Introduction
Navigating the intricate landscape of Good Manufacturing Practices (GMP) is essential for pharmaceutical companies striving for compliance and operational excellence. As the industry faces increasing scrutiny and evolving regulations, understanding and implementing effective GMP practices can significantly enhance product quality and safety.
However, with nearly 80% of medication shortages stemming from compliance issues, the challenge lies in identifying and addressing potential gaps in adherence. What essential practices can compliance officers adopt to not only meet regulatory requirements but also foster a culture of continuous improvement within their organizations?
By embracing a proactive approach to GMP, organizations can not only mitigate risks but also position themselves as leaders in quality assurance.
AVS Life Sciences: Comprehensive GMP Compliance Solutions for Pharmaceutical Companies
AVS Life Sciences offers a comprehensive suite of services designed to empower pharmaceutical companies in navigating the complexities of GMP practices. Their offerings encompass:
- Validation and commissioning
- Consulting for standards adherence
- Guidance on regulatory submissions, including GMP practices audits
All aimed at enabling clients to maintain exceptional excellence throughout the product lifecycle. By emphasizing innovation and expertise, AVS Life Sciences not only guarantees compliance with GMP practices but also enhances operational efficiency.
The execution of effective GMP practices is crucial, as nearly 80% of medication shortages arise from issues related to standards. Companies that adopt efficient management systems (QMS) can reap substantial operational benefits, including:
- A 40% reduction in customer complaints
- A 25% increase in customer retention rates
Recent trends highlight an increasing focus on continuous improvement and the integration of digital technologies, such as AI and machine learning, into management practices. These advancements are expected to revolutionize how pharmaceutical firms , ensuring that every batch of products meets stringent standards and regulatory requirements.
AVS Life Sciences sets itself apart in the industry by equipping clients with the necessary tools and knowledge to confront these evolving challenges, ultimately fostering a culture of excellence and compliance that is essential for success in the pharmaceutical sector. The FDA's new Quality Management Maturity (QMM) program, piloted with 15 manufacturers globally, underscores the shifting landscape of GMP practices compliance. Organizations that achieve strong QMM scores may benefit from fewer inspections and expedited regulatory reviews, further underscoring the advantages of implementing effective management systems.

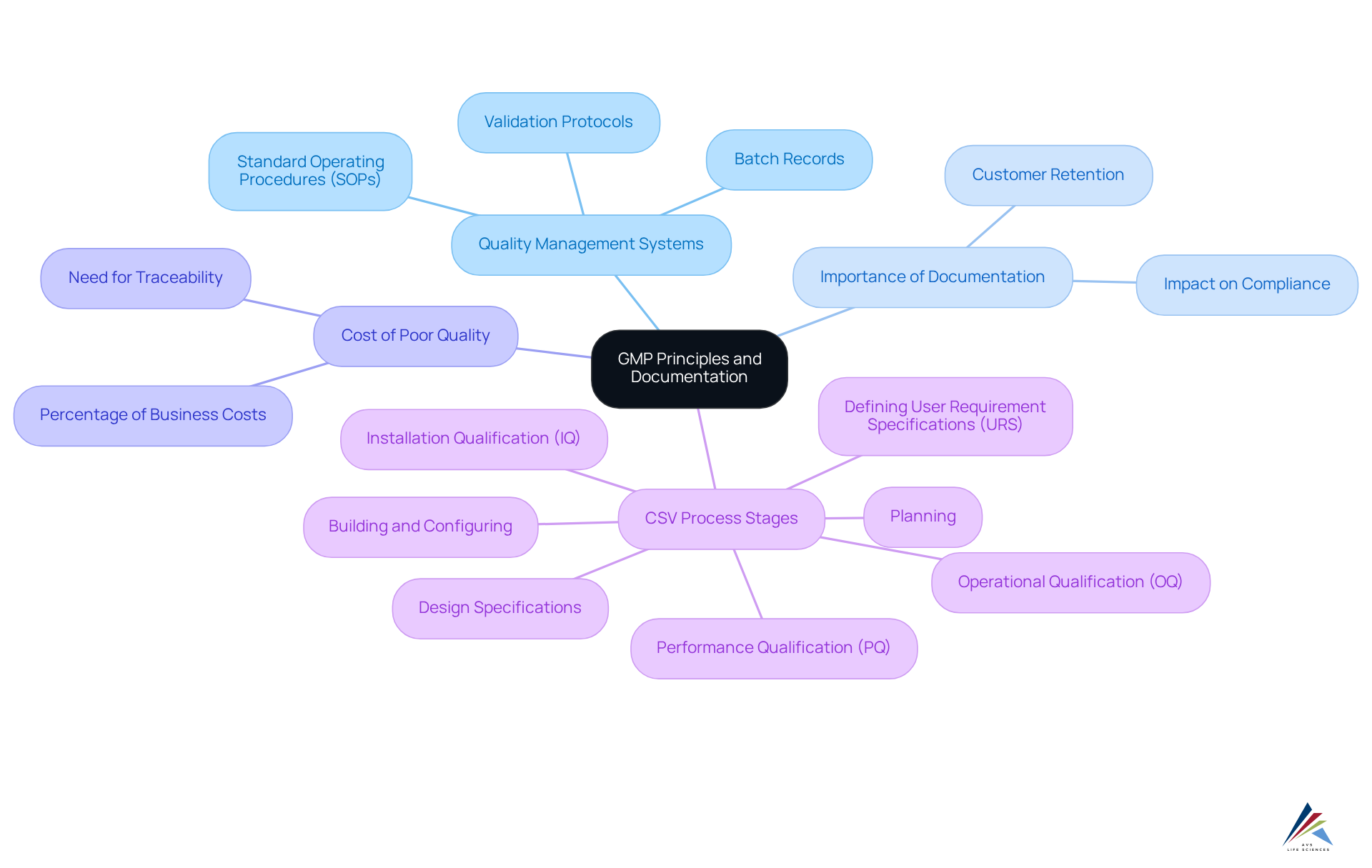
GMP Principles: Establishing Quality Management Systems and Documentation
GMP Principles: Establishing [Quality Management Systems](https://avslifesciences.com/about-us) and Documentation
GMP principles focus on establishing effective quality management systems (QMS) that guarantee products are consistently produced and controlled in accordance with stringent quality standards. Central to this framework is comprehensive documentation that delineates procedures, responsibilities, and processes. Key elements consist of Standard Operating Procedures (SOPs), batch records, and validation protocols, which are crucial for upholding regulations and ensuring product safety as part of GMP practices.
Importance of Documentation in GMP Compliance
In 2025, 60% of manufacturers believe a robust quality management system positively impacts product compliance and regulatory requirements. Organizations with mature QMS report a 25% higher customer retention rate, illustrating the direct correlation between effective documentation practices and GMP practices in achieving overall business success.
Cost of Poor Quality
The can range from 15% to 35% of total business costs in regulated industries, emphasizing the need for meticulous documentation to prevent errors and ensure traceability. Batch records must offer thorough details concerning the production and oversight of each batch, while SOPs are crucial for executing operational tasks in accordance with GMP practices.
Computer System Validation (CSV) Process Stages
- Planning: Determine necessary budget and timeline for CSV.
- Defining User Requirement Specifications (URS): Outline the tasks the system must perform.
- Design Specifications: Decide on the appearance and operation of functions.
- Building and Configuring the System: Develop configured scripts for software design.
- Installation Qualification (IQ): Execute scripts to verify installation methods.
- Operational Qualification (OQ): Test to ensure the system operates as intended.
- Performance Qualification (PQ): Simulate worst-case scenarios to ensure functionality.
As the pharmaceutical sector keeps advancing, the focus on documentation methods will only grow stronger, making it essential for oversight officers to prioritize the creation and upkeep of clear, concise, and logical records to satisfy regulatory requirements and protect public health.
Training Programs: Implementing Effective GMP Training for Staff
Establishing effective [GMP practices](https://avslifesciences.com/job-opportunities/qa-validation) in training programs is essential for ensuring that employees are well-informed about their roles and responsibilities regarding adherence. Training should cover key areas such as:
- Proper documentation practices
- Equipment handling
- Sanitation procedures
Regular refresher courses are crucial; adherence metrics indicate that organizations with robust training programs experience a 30% decrease in product recalls. Furthermore, evaluations must be integrated to enhance understanding and adapt to evolving regulations, thereby fostering a culture of adherence within the organization. Current trends underscore a growing emphasis on competency-based training models, aligning employee skills with GMP practices and the organization's objectives.
Successful examples, such as AVS Life Sciences' collaboration with a leading biotechnology company to upgrade their GMP facility, illustrate the effectiveness of hands-on training modules that engage employees with real-world scenarios. This initiative not only improved operational efficiency but also ensured strict compliance with quality assurance protocols, ultimately preserving high standards of quality and adherence.

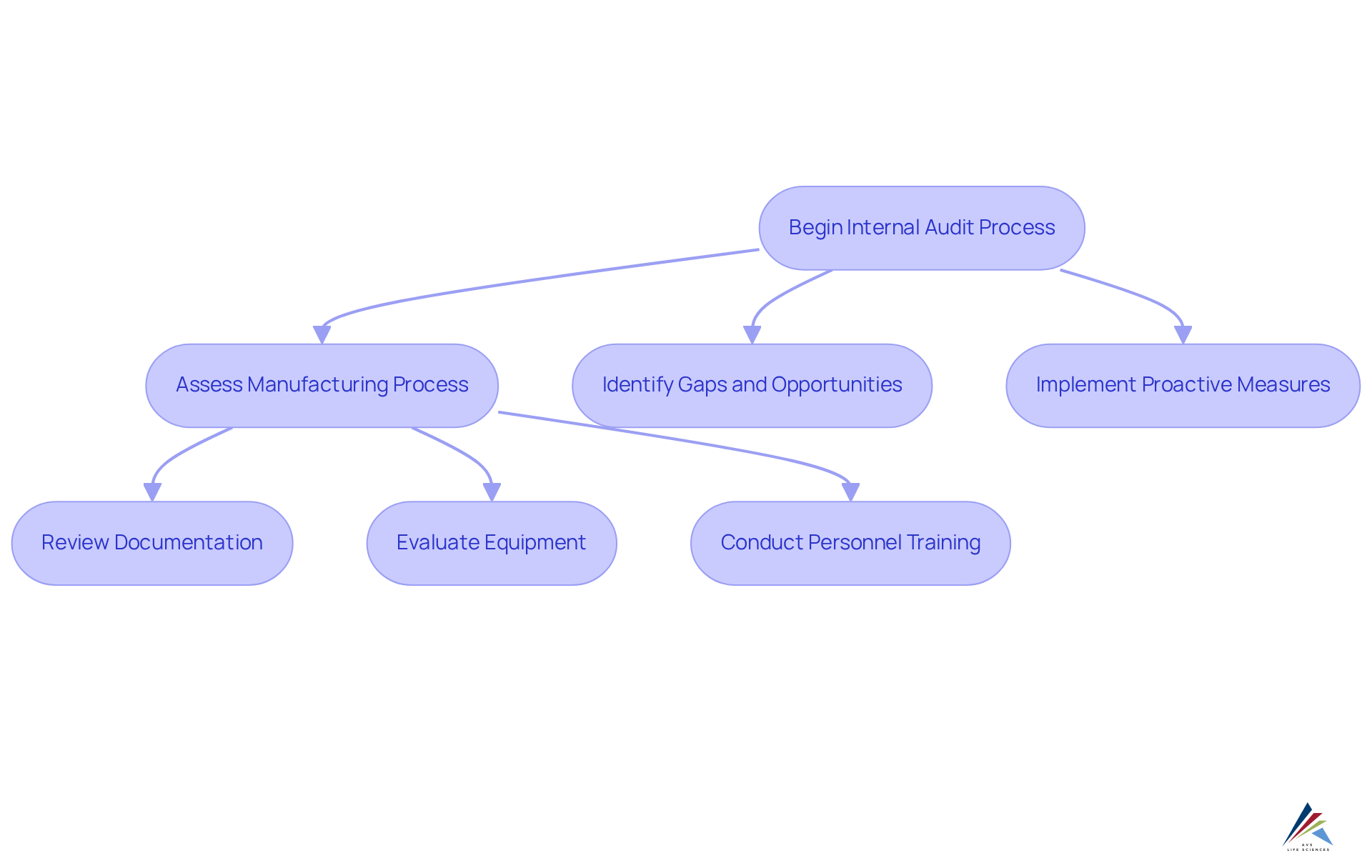
Internal Audits: Ensuring Continuous Compliance with GMP Standards
Regular internal audits are crucial for maintaining compliance with GMP practices standards within the pharmaceutical sector. These audits must thoroughly assess every aspect of the manufacturing process, including:
- Detailed documentation
- Equipment upkeep
- Personnel training
By systematically pinpointing gaps and opportunities for enhancement, organizations can proactively resolve regulatory concerns before they escalate, ensuring they are consistently audit-ready and in alignment with regulatory requirements. Recent studies indicate that companies with robust internal audit programs demonstrate significantly higher compliance rates, underscoring the importance of these evaluations in fostering a culture of quality and accountability.
Effective internal audits should incorporate GMP practices such as:
- Utilizing technology for data analytics
- Providing comprehensive training for audit staff
- Fostering open communication across departments
This proactive approach not only enhances compliance but also contributes to the overall efficiency and dependability of pharmaceutical operations.
Equipment Maintenance: Ensuring Compliance Through Proper Equipment Management
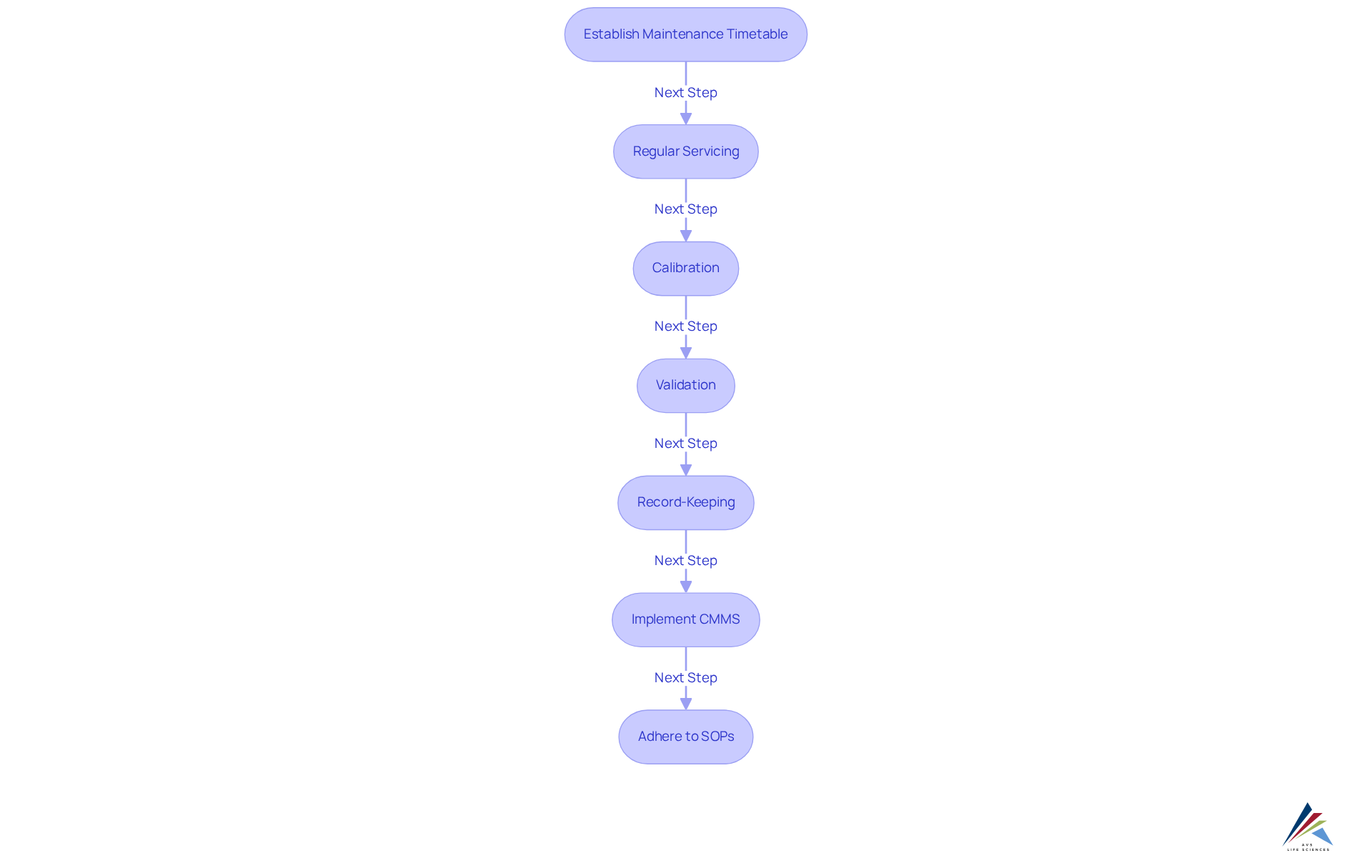
Effective equipment maintenance is crucial for achieving GMP practices in the pharmaceutical industry. Regular servicing, calibration, and validation of equipment are fundamental to ensuring consistent and reliable operation, in accordance with GXP standards and FDA regulations. Establishing a comprehensive maintenance timetable, alongside meticulous recording of all maintenance tasks, is vital for demonstrating adherence during audits. This process includes compliance with Standard Operating Procedures (SOPs) and safeguarding data integrity throughout maintenance activities.
Implementing a Computerized Maintenance Management System (CMMS) significantly bolsters these efforts by streamlining maintenance processes, automating record-keeping, and ensuring that documentation is precise and readily accessible. This system not only facilitates adherence to industry standards but also supports , effectively reducing the risk of unexpected downtime and costly repairs. A well-executed CMMS can track calibration schedules and validate maintenance actions, reinforcing the integrity of the equipment management process.
By prioritizing GMP practices, organizations can adeptly manage their equipment, ensuring both operational efficiency and compliance with GMP regulations.
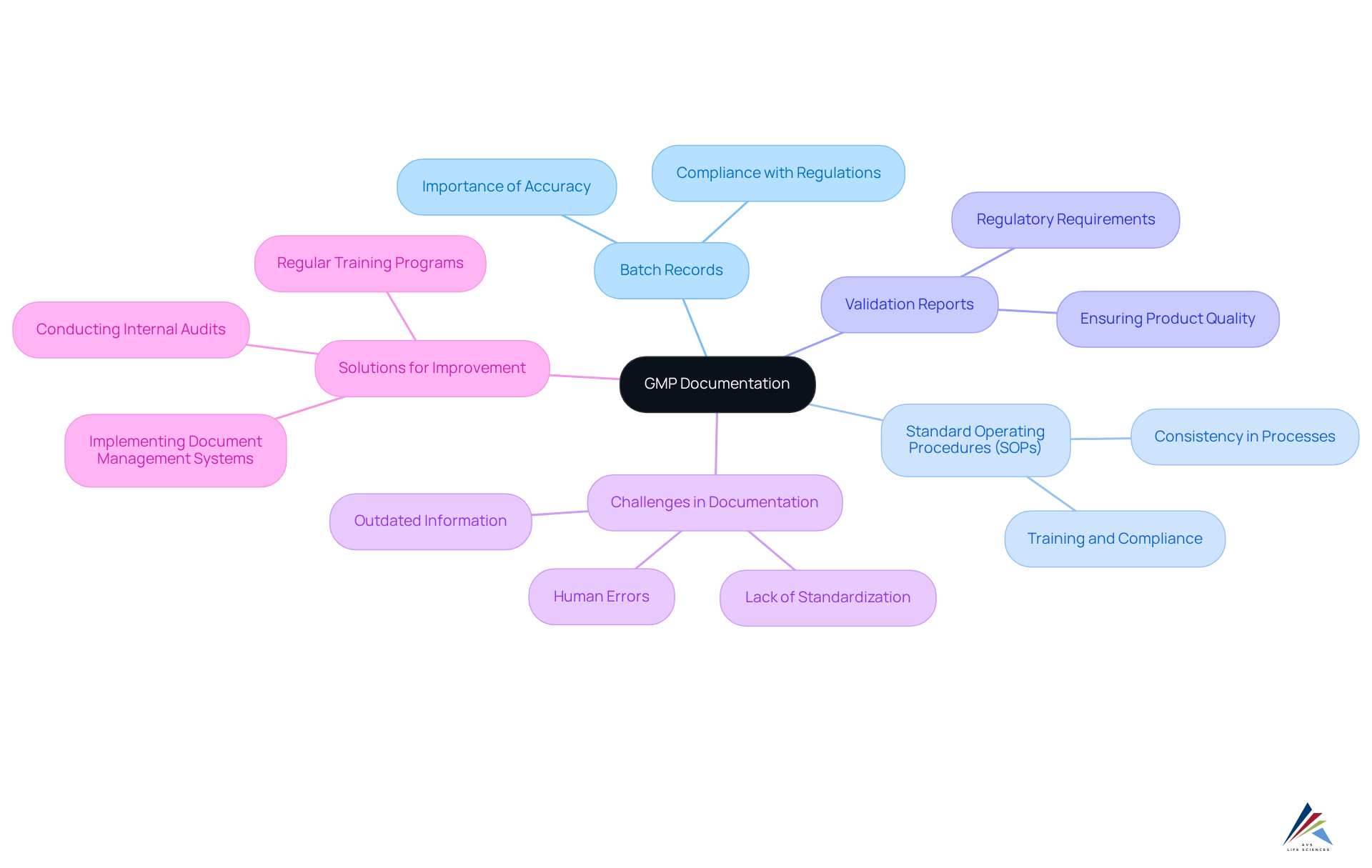
Documentation: Maintaining Accurate Records for GMP Compliance
Precise record-keeping is essential for ensuring compliance with GMP practices. Documentation must encompass every aspect of the manufacturing process, from the sourcing of raw materials to the testing of final products. Key documents integral to this process include:
- Batch records
- Standard Operating Procedures (SOPs)
- Validation reports
Establishing a robust document control system is vital; it ensures that all records are current, easily accessible, and aligned with regulatory standards. This not only promotes transparency and accountability but also mitigates the risk of . Recent trends indicate a shift towards automated document management solutions, which enhance efficiency and minimize human error, further supporting the integrity of documentation practices in the pharmaceutical sector.
Quality Control: Establishing Effective Quality Control Systems in GMP
Creating efficient control systems is essential for ensuring that pharmaceutical products meet safety and efficacy standards. A robust quality control (QC) framework encompasses rigorous testing of raw materials, in-process controls, and comprehensive evaluations of final products. The recent case study of AVS Life Sciences exemplifies this necessity, showcasing their successful upgrade of a biotechnology GMP facility from Level 1 to Level 2. This transformation adhered to strict assurance standards and underscored the throughout the process.
During the upgrade, specific challenges emerged, notably anomalies in test results caused by barcode scanner cameras being installed upside down. This oversight led to the technician receiving misleading outcomes, initially recorded as 'Passed.' The implementation of statistical process control (SPC) methods proved beneficial, enabling continuous monitoring of production processes to identify variations that could compromise product standards. SPC tools facilitated real-time tracking of critical process parameters, allowing for immediate corrective actions before deviations escalated into compliance issues.
Current trends in control emphasize a proactive approach, where organizations leverage data analytics to enhance decision-making and risk management. Recent advancements in 2025 have witnessed the adoption of innovative technologies that streamline QC processes, such as automated sampling and advanced statistical analysis, which improve the precision and efficiency of assessments.
Examples of effective QC processes that align with GMP practices include:
- Establishing clear specification acceptance criteria for raw materials and finished products
- Conducting regular audits to ensure conformity to established quality standards
By fostering a culture of continuous improvement and utilizing SPC, organizations can significantly enhance their GMP practices, reduce variability, and ultimately deliver high-quality pharmaceutical products that meet regulatory expectations. The FDA underscores the necessity for pharmaceutical producers to deepen their understanding of GMP-related statistics, further emphasizing the critical role of SPC in maintaining standards.

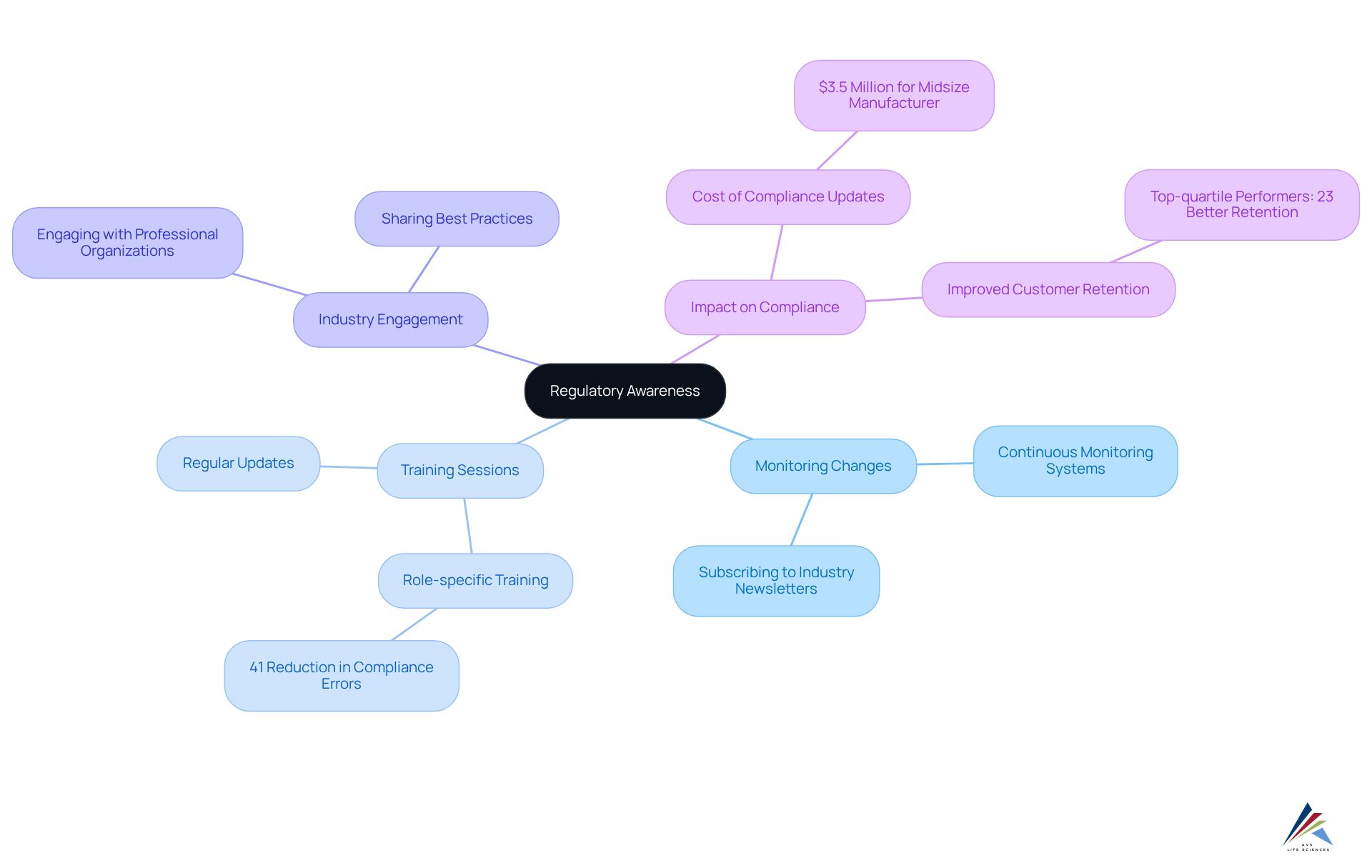
Regulatory Awareness: Keeping Up with GMP Regulations and Changes
Regulatory awareness is paramount for ensuring adherence to GMP practices. Organizations must establish robust systems to monitor changes in regulations and guidelines from authorities such as the FDA and EMA. Regular training sessions are crucial; they keep staff informed about the latest requirements, fostering a culture of compliance. For example, companies that implemented role-specific training programs reported a 41% reduction in compliance errors, showcasing the effectiveness of targeted education.
AVS Life Sciences underscores the importance of subscribing to industry newsletters and engaging with professional organizations to gain valuable insights into emerging trends and compliance changes. The Korean pharmaceutical sector, for instance, has significantly enhanced its international competitiveness by proactively adapting to , exemplifying the benefits of remaining informed. By prioritizing regulatory awareness and implementing GMP practices, pharmaceutical firms can elevate their adherence rates and navigate the complexities of the industry more effectively. Notably, top-quartile adherence performers exhibit 23% better customer retention than their bottom-quartile counterparts, reinforcing the business case for robust adherence practices. Regulatory officers are thus encouraged to implement continuous monitoring systems to stay ahead of legal changes and ensure ongoing compliance.
Consulting Services: Leveraging External Expertise for GMP Compliance
Utilizing outside can significantly enhance an organization's adherence to GMP practices. Consultants bring specialized knowledge and extensive experience, enabling them to identify regulatory gaps and develop tailored improvement strategies. Their expertise encompasses training, documentation, and conducting internal audits, providing an objective perspective that often leads to more effective regulatory solutions.
A notable example of this is AVS Life Sciences' recent collaboration with a leading biotechnology company in San Francisco, where they successfully upgraded the client's manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. This project included a thorough gap analysis and highlighted critical lessons learned, such as the oversight of barcode scanner installations, which initially resulted in unreliable test outcomes. The project was completed on time and within budget, exemplifying AVS's commitment to assurance and compliance with industry standards.
By partnering with specialists like AVS Life Sciences, organizations can ensure alignment with industry best practices and compliance expectations, fostering a culture of quality throughout all operational levels. Recent trends indicate a growing reliance on consulting services, particularly as the pharmaceutical industry faces increased oversight and demands for innovative solutions. The global regulatory consulting outsourcing services market is projected to reach USD 1.90 billion by 2030, underscoring the rising demand for these services.
Effective partnerships, such as AVS Life Sciences' introduction of robust Quality Management Systems (QMS), demonstrate how consultants can optimize processes and enhance operational efficiency, ultimately promoting adherence and product integrity. Organizations should regularly assess their adherence metrics to evaluate the effectiveness of their consulting partnerships, ensuring continuous improvement in their GMP practices.
Common Shortcomings: Identifying and Addressing GMP Compliance Gaps
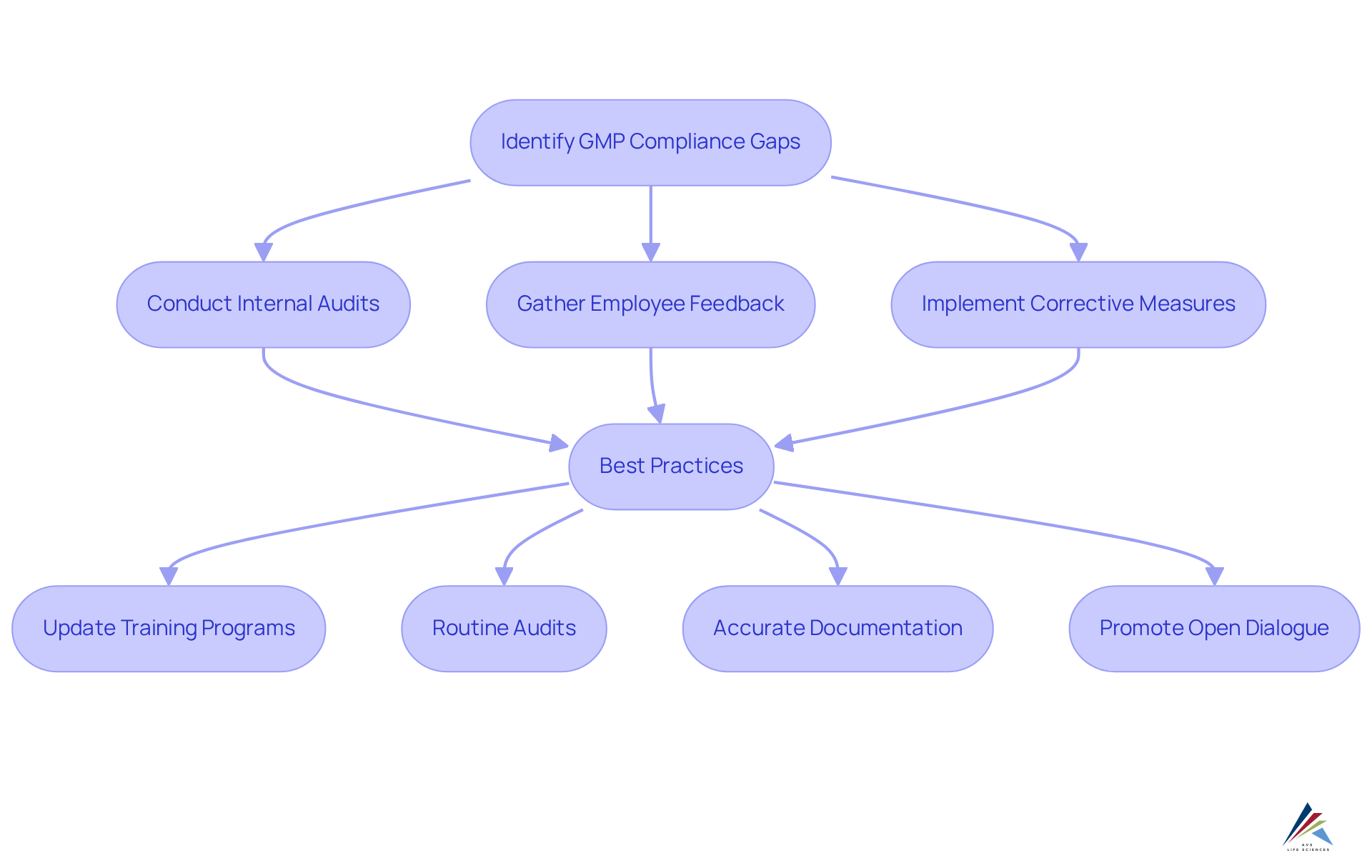
Recognizing shared deficiencies in GMP practices adherence is essential for fostering a culture of continuous improvement. Common challenges include inadequate documentation, insufficient training, and a lack of regular audits, which can result in substantial regulatory violations. Notably, the FDA has reported a , underscoring the urgent need for organizations to proactively address these gaps.
To effectively pinpoint these shortcomings, organizations must conduct comprehensive assessments that encompass regular internal audits and employee feedback mechanisms. Implementing corrective measures based on these evaluations is crucial for maintaining standards and enhancing overall management systems.
Establishing an environment of continuous improvement, where feedback is actively encouraged and addressed, can significantly bolster adherence efforts. Companies that prioritize ongoing training and development for their staff are better equipped to mitigate risks associated with GMP practices violations. Furthermore, integrating assurance practices within production processes fosters a collaborative atmosphere that enhances problem-solving capabilities.
Best practices for addressing GMP compliance gaps include:
- Regularly updating training programs to reflect current regulations and industry standards.
- Conducting routine audits to ensure adherence to established protocols.
- Maintaining accurate and comprehensive documentation to facilitate traceability and accountability.
- Promoting open dialogue between the standards and production teams to swiftly identify and resolve issues.
By committing to continuous improvement in GMP practices, organizations can enhance compliance, elevate product quality and safety, ultimately leading to improved outcomes in the pharmaceutical manufacturing landscape.
Conclusion
Implementing effective Good Manufacturing Practices (GMP) is essential for pharmaceutical companies striving for compliance and excellence. This imperative not only ensures regulatory adherence but also enhances operational efficiency and product quality. By focusing on robust quality management systems, meticulous documentation, and continuous staff training, organizations can significantly reduce risks associated with non-compliance and improve overall performance.
Key insights discussed include:
- The necessity of thorough documentation
- The role of internal audits in maintaining compliance
- The value of external consulting services to identify and address regulatory gaps
Furthermore, the impact of innovative technologies and training programs on fostering a culture of quality and accountability within the industry is paramount. With nearly 80% of medication shortages linked to GMP-related issues, the urgency for pharmaceutical companies to prioritize these practices cannot be overstated.
In conclusion, the commitment to continuous improvement in GMP practices is vital for the pharmaceutical sector. Organizations are encouraged to adopt a proactive approach, leveraging training, technology, and external expertise to navigate the complexities of compliance effectively. By doing so, they not only safeguard public health but also enhance their competitive edge in a rapidly evolving industry landscape. Embracing these essential GMP practices will ultimately lead to higher quality products, reduced regulatory violations, and increased customer satisfaction, reinforcing the significance of adherence in the pharmaceutical manufacturing process.
Frequently Asked Questions
What services does AVS Life Sciences provide to pharmaceutical companies?
AVS Life Sciences offers services such as validation and commissioning, consulting for standards adherence, and guidance on regulatory submissions, including GMP practices audits, all aimed at helping clients maintain excellence throughout the product lifecycle.
Why are effective GMP practices important for pharmaceutical companies?
Effective GMP practices are crucial because nearly 80% of medication shortages arise from issues related to standards. Companies that adopt efficient management systems can experience significant operational benefits, including a 40% reduction in customer complaints and a 25% increase in customer retention rates.
What recent trends are influencing GMP practices in the pharmaceutical industry?
Recent trends include a focus on continuous improvement and the integration of digital technologies, such as AI and machine learning, into management practices, which are expected to revolutionize how pharmaceutical firms manage GMP compliance.
How does AVS Life Sciences differentiate itself in the industry?
AVS Life Sciences differentiates itself by equipping clients with the necessary tools and knowledge to tackle evolving challenges, fostering a culture of excellence and compliance essential for success in the pharmaceutical sector.
What is the FDA's Quality Management Maturity (QMM) program?
The FDA's QMM program, piloted with 15 manufacturers globally, aims to improve GMP practices compliance. Organizations that achieve strong QMM scores may benefit from fewer inspections and expedited regulatory reviews.
What are the key elements of GMP principles regarding quality management systems?
Key elements of GMP principles include comprehensive documentation, Standard Operating Procedures (SOPs), batch records, and validation protocols, which are essential for upholding regulations and ensuring product safety.
What is the cost of poor quality in regulated industries?
The cost of poor quality can range from 15% to 35% of total business costs in regulated industries, highlighting the importance of meticulous documentation to prevent errors and ensure traceability.
What are the stages of the Computer System Validation (CSV) process?
The stages of the CSV process include: Planning, Defining User Requirement Specifications (URS), Design Specifications, Building and Configuring the System, Installation Qualification (IQ), Operational Qualification (OQ), Performance Qualification (PQ).
Why is training important for GMP compliance?
Training is essential for ensuring that employees understand their roles and responsibilities regarding GMP adherence. Organizations with robust training programs experience a 30% decrease in product recalls.
What current trends are observed in GMP training programs?
There is a growing emphasis on competency-based training models, which align employee skills with GMP practices and the organization's objectives, ensuring effective adherence to regulations.
