10 CTMS Clinical Trial Benefits for Compliance Officers

Overview
The article delineates ten pivotal benefits of Clinical Trial Management Systems (CTMS) specifically tailored for compliance officers, accentuating how these systems bolster regulatory adherence, operational efficiency, and data integrity in clinical trials. It elaborates on features such as:
- Automated reporting
- Real-time data access
- Streamlined documentation processes
Collectively, these functionalities alleviate administrative burdens and enhance compliance outcomes, ultimately culminating in more successful trial results. By addressing the compliance challenges faced by professionals in the field, the article not only highlights the advantages of CTMS but also positions these solutions as essential tools for achieving excellence in clinical trial management.
Introduction
The landscape of clinical trials is evolving rapidly, presenting compliance officers with increasing pressure to navigate a complex web of regulations while ensuring the integrity of their research.
With the demand for Clinical Trial Management Systems (CTMS) on the rise—particularly as the market is projected to reach USD 7.9 billion by 2033—understanding the multifaceted benefits of these systems becomes essential.
Compliance officers encounter numerous challenges in this dynamic environment; however, CTMS can be the key to overcoming them.
This article explores ten critical advantages of CTMS tailored specifically for compliance officers, revealing how these systems can streamline processes, enhance data quality, and ultimately lead to more successful clinical trials.
AVS Life Sciences: Comprehensive CTMS Solutions for Regulatory Compliance
AVS Life Sciences excels in providing tailored solutions for CTMS clinical trial management specifically designed for the pharmaceutical and biotechnology sectors. These services are crucial for navigating the complexities of regulatory environments, ensuring compliance with Good Manufacturing Practices (GMP) and Quality System Regulations (QSR). AVS Life Sciences empowers organizations to enhance their clinical research procedures while upholding rigorous standards, including robust documentation practices, information integrity measures, and adherence to CAPA FDA regulations.
As the CTMS clinical trial market is projected to reach USD 7.9 billion by 2033, the demand for effective clinical trial management solutions is expanding significantly. The evolving landscape of CTMS clinical trial management systems underscores the integration of cutting-edge technologies that enhance information handling and operational effectiveness. The implementation of a CTMS clinical trial management system not only supports regulatory compliance but also significantly reduces administrative burdens, allowing research teams to concentrate on patient care and information integrity. By adopting these innovative solutions, organizations can improve oversight and ensure timely project delivery, which is vital in today’s competitive environment.
The benefits of CTMS clinical trial management systems extend beyond mere compliance; they promote enhanced collaboration among stakeholders, improved information accuracy, and the ability to adapt to the increasing complexity of clinical trials. As the pharmaceutical industry continues to invest heavily in research and development, the role of CTMS clinical trial management systems in facilitating regulatory compliance and boosting efficiency becomes increasingly critical. AVS Life Sciences remains dedicated to delivering comprehensive solutions that address the evolving needs of its clients, positioning them as leaders in the life sciences consulting market.
Enhanced Data Quality and Integrity: A Core Benefit of CTMS
A major benefit of adopting a Clinical Trial Management System is its substantial enhancement of information quality and integrity. By centralizing information management, CTMS platforms facilitate real-time monitoring and validation of entries, which is crucial for maintaining high standards in clinical research. The automation within these systems significantly reduces the risk of human error by minimizing manual input and automating validation processes, ensuring that the information gathered during clinical trials is both accurate and dependable. This level of information integrity is essential not only for complying with regulatory standards, such as GXP and FDA regulations, but also for fostering trust among stakeholders involved in the CTMS clinical trial.
Real-world instances underscore the effectiveness of clinical trial management systems in enhancing information integrity. For instance, automated Electronic Information Capture (EDC) systems, when integrated with CTMS, have streamlined information collection processes, allowing researchers to focus more on analysis rather than paperwork. Furthermore, the principles of information integrity, encapsulated in the ALCOA framework—Attributable, Legible, Contemporaneous, Original, and Accurate—are upheld through automated checks and balances, ensuring adherence to ethical standards in clinical studies. The expanded edition, ALCOA+, which includes additional principles such as Complete, Consistent, Enduring, and Available, further emphasizes the complexity of information management in clinical studies.
In 2025, the importance of information integrity in clinical trial management cannot be overstated. Regulatory bodies enforce compliance with stringent information integrity standards, and nonconformity can lead to the denial of marketing authorization applications. CTMS enhances information management and validation by promoting uniform entry methods and automating verification processes, which together bolster the reliability of the information collected. This proactive approach to data integrity not only safeguards patient safety but also supports the ethical conduct of clinical research. Moreover, the comprehensive computer system validation procedure, which includes phases such as Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ), ensures that the systems employed in clinical studies comply with all necessary standards.
Increased Efficiency in Clinical Trial Management Through CTMS
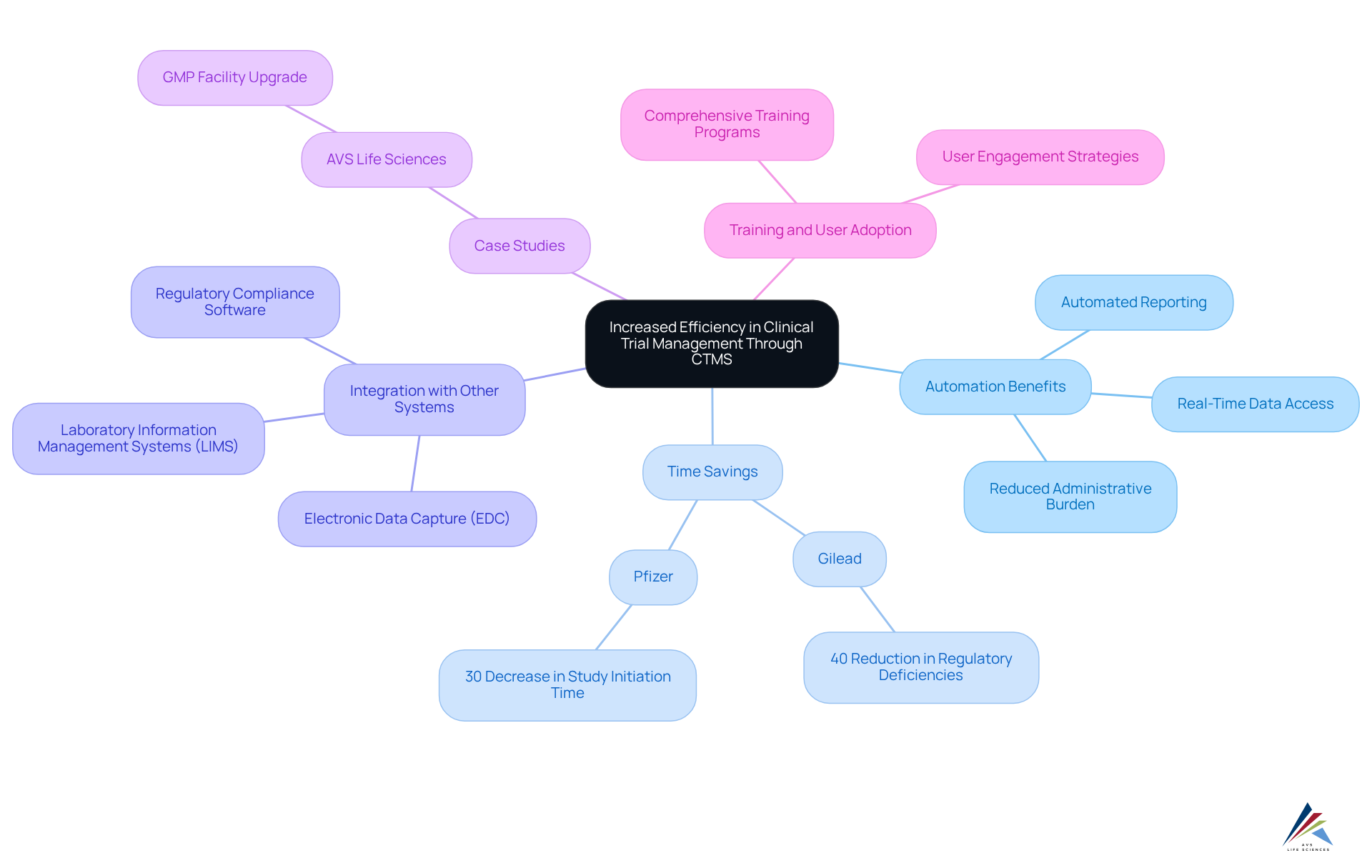
Clinical Study Management Systems significantly enhance efficiency in clinical study oversight by automating crucial tasks, including scheduling, patient tracking, and document management. This automation alleviates the administrative burden on clinical research teams, allowing them to focus on critical activities that require human oversight.
Companies that have implemented clinical management systems report substantial time savings; for instance:
- Gilead noted a 40% reduction in regulatory deficiencies.
- Pfizer experienced a 30% decrease in study initiation time.
By optimizing workflows, these systems facilitate faster decision-making and expedite the entire trial schedule, which is essential for meeting regulatory deadlines. Furthermore, integrating clinical trial management systems with platforms such as Electronic Data Capture (EDC) and Laboratory Information Management Systems (LIMS) enhances information quality and minimizes inconsistencies, ultimately leading to more reliable outcomes.
Key features of the system, such as automated reporting and real-time data access, are crucial for ensuring compliance and improving quality control processes. A recent case study illustrates this:
- AVS Life Sciences successfully assisted a leading biotechnology company in upgrading their GMP facility, significantly enhancing their quality control processes. This upgrade not only ensured compliance with stringent regulations but also allowed the client to concentrate on developing innovative medicines.
Such examples underscore the critical role of quality management systems in clinical research. As the clinical research landscape evolves in 2025, the automation advantages of clinical trial management systems will continue to be pivotal in improving trial administration for oversight officers. Comprehensive training programs are essential for ensuring staff proficiency in utilizing clinical trial management system features, thereby maximizing the potential benefits of these systems.
Streamlined Regulatory Compliance: How CTMS Simplifies Processes
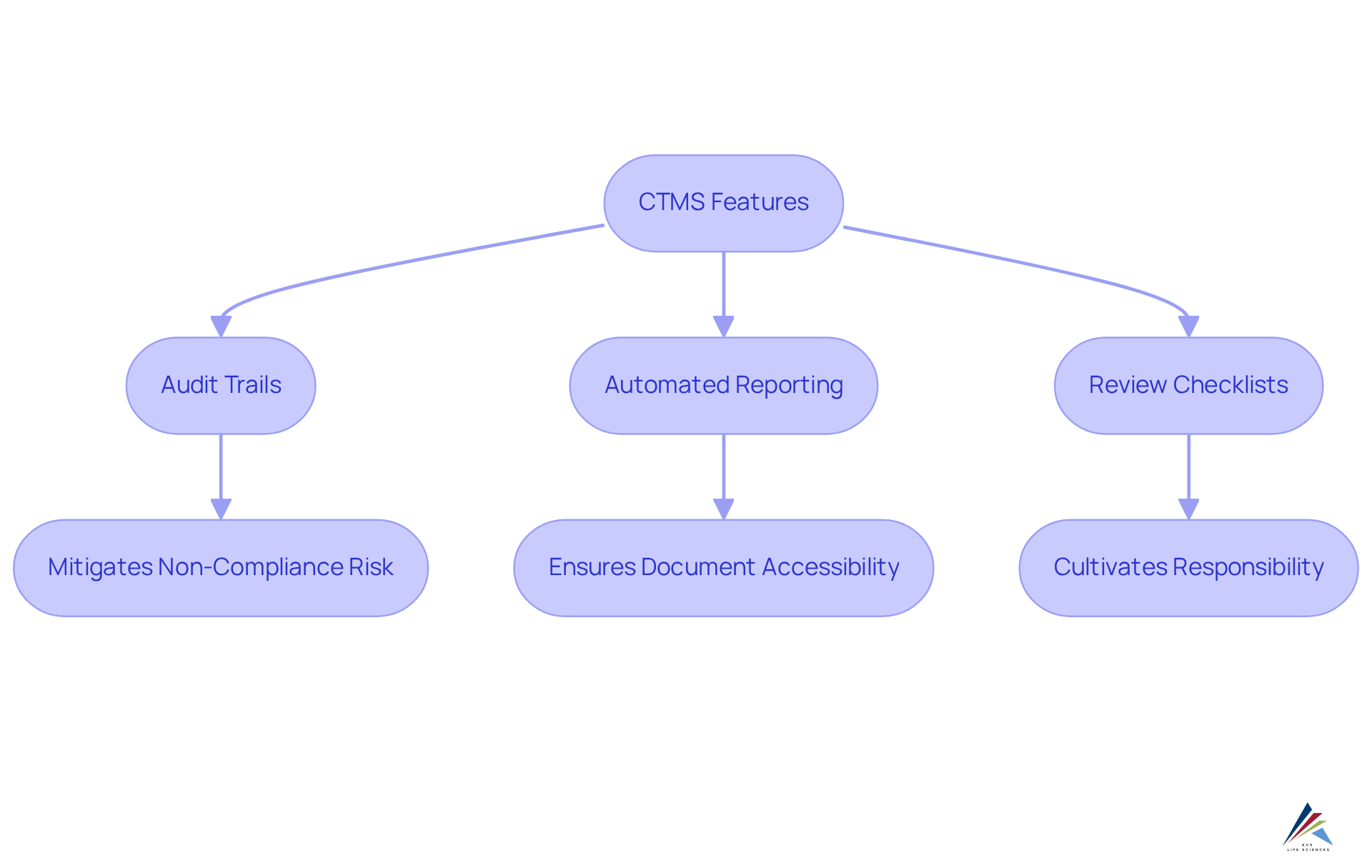
Management systems are meticulously crafted to enhance regulatory adherence by integrating essential features such as audit trails, review checklists, and automated reporting. These functionalities ensure that all critical documentation is meticulously preserved and readily accessible during audits, significantly mitigating the risk of non-compliance. Such risks can lead to severe consequences, including legal suspension, financial penalties, and reputational damage. Notably, research indicates that over 70% of entities utilizing CTMS clinical trial report improved adherence outcomes, underscoring the system's effectiveness in navigating complex regulatory landscapes.
By optimizing the adherence process, the system not only aids organizations in avoiding potential pitfalls but also ensures compliance with regulatory standards throughout the study lifecycle. For instance, the automated version control feature guarantees that only the latest authorized documents are utilized, while previous versions are securely archived. This practice upholds the integrity of clinical trial documentation and facilitates organized records and tracking of adherence.
Moreover, seamless integration with other systems, such as electronic data capture (EDC) and laboratory information management systems (LIMS), is crucial for the effective operation of CTMS clinical trial. This integration enhances workflow efficiency and ensures that all data remains synchronized, further supporting regulatory compliance efforts.
Real-world scenarios demonstrate how audit trails within CTMS clinical trial management systems streamline regulatory inspections by providing immediate access to vital documents, thereby fostering transparency and accountability. Additionally, adherence checklists serve as indispensable tools for research teams, cultivating a culture of responsibility regarding regulatory standards. In summary, the robust capabilities of these systems significantly enhance regulatory processes, making them indispensable for organizations committed to maintaining high compliance standards in clinical research.
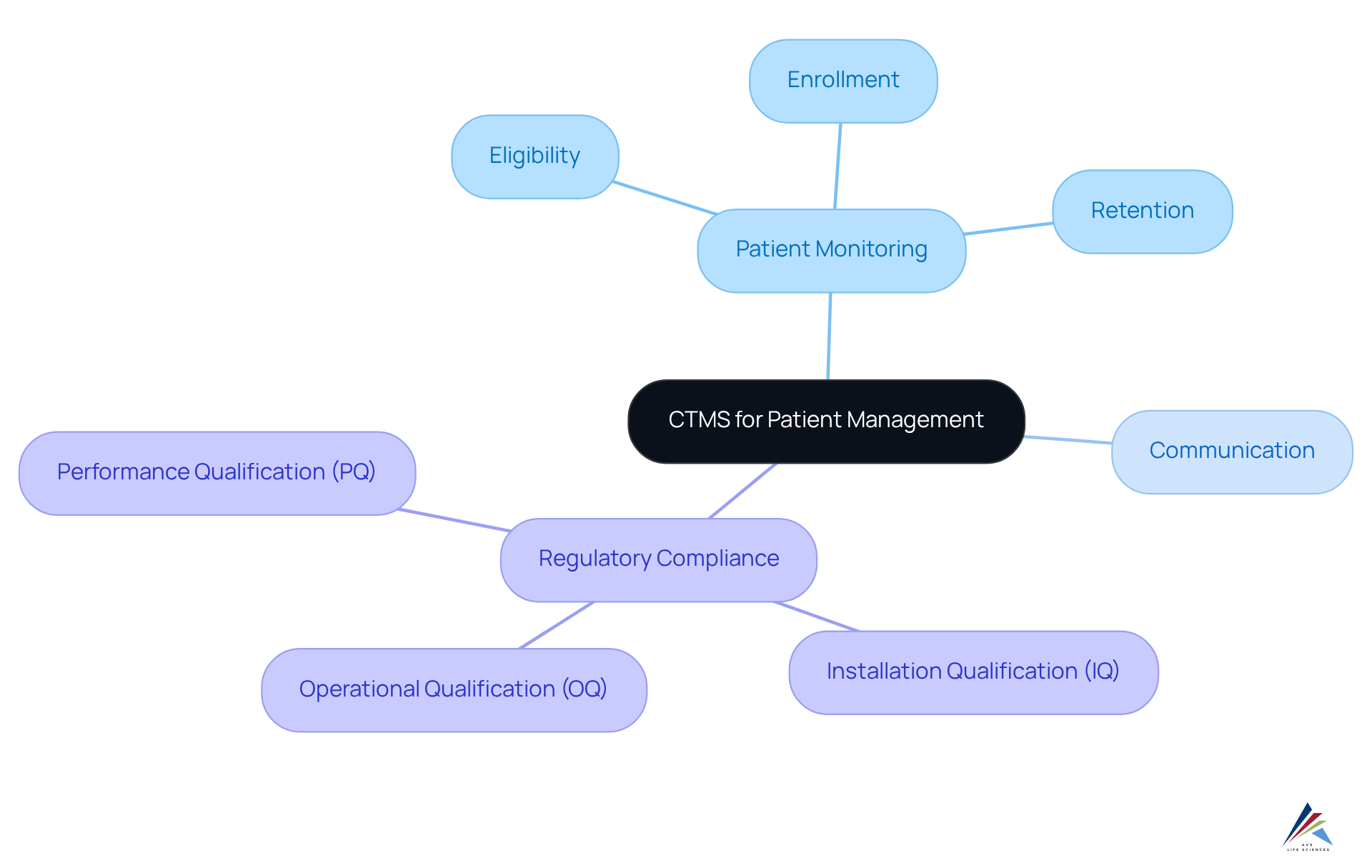
Improved Patient Management: Leveraging CTMS for Better Outcomes
The system enhances patient management by providing essential resources for monitoring patient eligibility, enrollment, and retention throughout the study. It features real-time updates on patient status and facilitates communication, enabling clinical teams to respond swiftly to patient needs. This proactive strategy not only elevates patient satisfaction but also plays a crucial role in achieving higher retention rates, which are vital for the integrity of trial results.
Moreover, the integration of robust Computer System Validation (CSV) processes within CTMS guarantees compliance with regulatory standards, thereby strengthening the overall quality management framework. By adhering to the stages of CSV—Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ)—CTMS systems are validated to operate as intended, ensuring accurate and secure management of patient information.
This commitment to adherence and quality assurance is indispensable for pharmaceutical regulatory officers dedicated to upholding the integrity of clinical research.
Real-Time Data Access and Reporting: Key Advantages of CTMS
A defining advantage of CTMS clinical trial is their provision of real-time information access and reporting. This functionality empowers regulatory officers and clinical teams to consistently oversee study progress, facilitating the early detection of problems and informed decision-making based on the most recent data. Immediate reporting not only enhances transparency and accountability but also ensures that all parties remain informed and aligned with the objectives and regulatory requirements.
In fact, a substantial percentage of regulatory officers—over 70%—are now utilizing real-time reporting tools within the CTMS clinical trial system to bolster their oversight processes. This trend underscores the increasing significance of immediate information access in upholding regulatory standards and enhancing study results in 2025.
For instance, AVS Life Sciences effectively assisted a prominent biotechnology firm in improving their GMP facility, demonstrating how efficient quality management methods—such as robust documentation practices and addressing data integrity issues—can significantly enhance monitoring and decision-making. This upgrade not only ensured compliance with FDA regulations but also elevated the overall quality assurance processes.
Furthermore, Roche's integration of a clinical management system enabled them to achieve substantial clinical study throughput, while Pfizer reported a significant reduction in study start-up time following the implementation of their clinical management system. These examples further illustrate the operational efficiencies realized through real-time reporting.
Cost-Effectiveness: Financial Benefits of Using CTMS
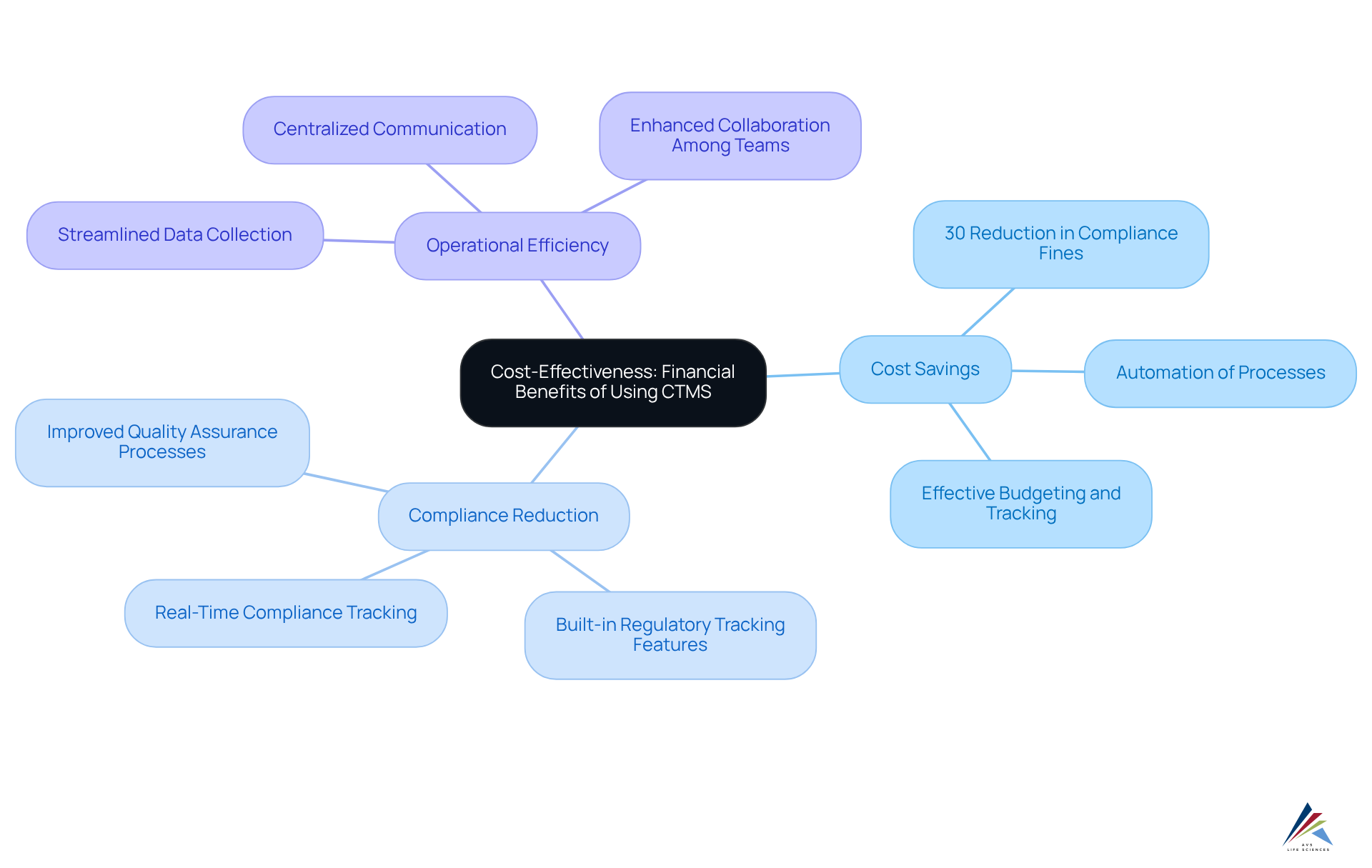
Implementing a CTMS clinical trial can yield substantial cost savings by streamlining operations and significantly reducing the risk of compliance-related penalties. By automating routine processes and improving data precision, the system allows organizations to allocate resources more effectively, consequently reducing the total costs related to CTMS clinical trials. Research indicates that organizations utilizing the CTMS clinical trial have experienced an average reduction in compliance-related fines of up to 30%, underscoring the financial benefits of this technology. For regulatory officers, who must navigate the complexities of adherence while managing budget constraints, these financial advantages are particularly compelling.
A transformative case study involving AVS Life Sciences illustrates this point effectively. The company assisted a leading biotechnology firm in upgrading their manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. This upgrade not only guaranteed adherence to stringent regulatory standards but also improved the quality assurance processes within the organization. The meticulous documentation and traceability efforts by AVS Life Sciences were recognized as appropriate by the client’s quality assurance team, showcasing the importance of comprehensive quality management in clinical research.
Essential aspects of the CTMS clinical trial management software, such as study protocol oversight, site administration, and data analysis, enable careful monitoring of compliance metrics, allowing for proactive modifications that avert expensive penalties and enhance overall study efficiency. Moreover, the integration features of the system with other platforms simplify processes, ensuring that all required documentation and approvals are finalized correctly and promptly.
As Michael Young, Co-CEO, states, "The application of Clinical Study Management System software has transformed the administration of clinical studies." This blend of operational efficiency and regulatory adherence establishes the system as a vital resource for organizations seeking to enhance their CTMS clinical trial costs.
Actionable Insights:
- Leverage CTMS features for real-time compliance tracking.
- Utilize data analytics to identify trends and prevent protocol deviations.
- Integrate the clinical trial management system with existing systems for enhanced efficiency.
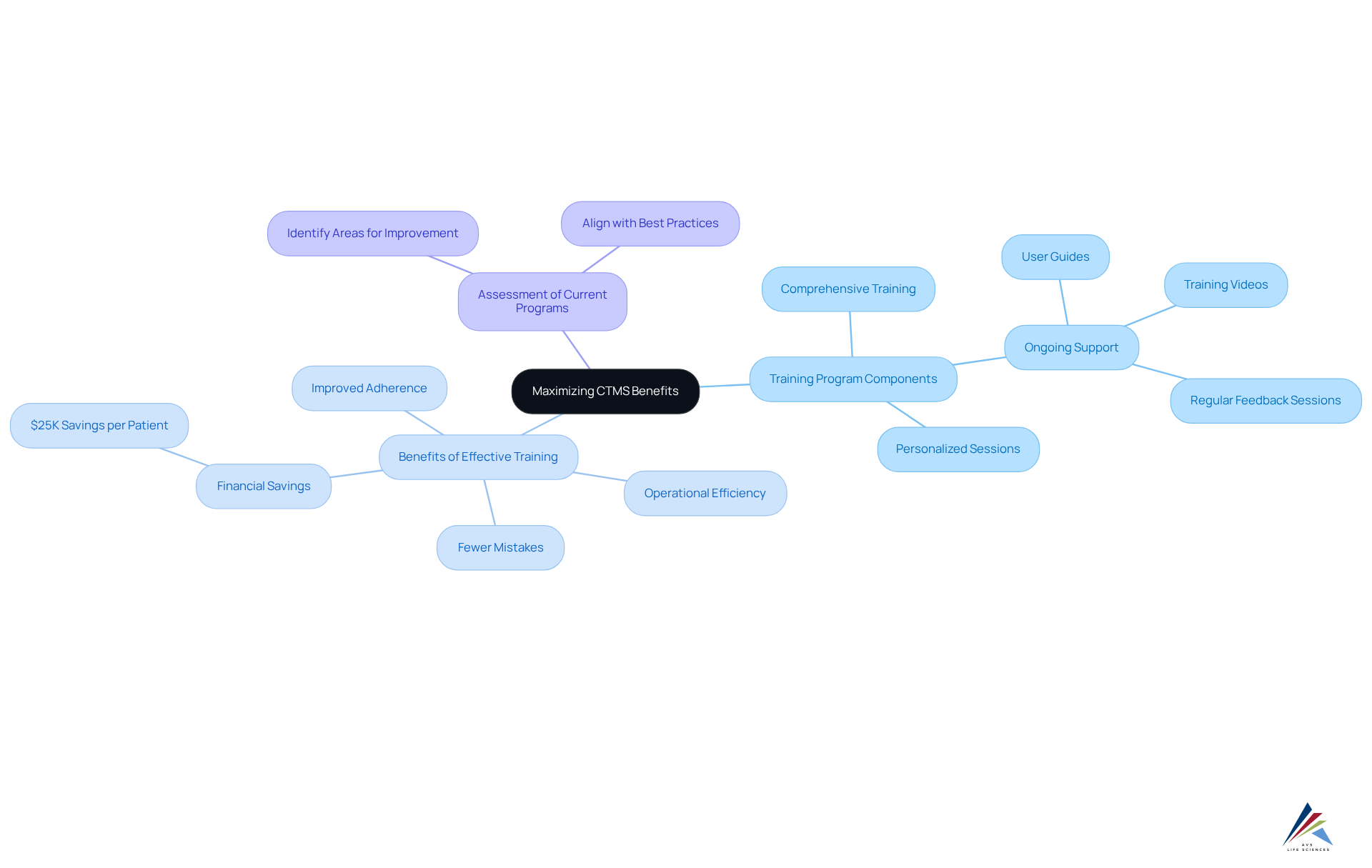
Effective Training and User Adoption: Maximizing CTMS Benefits
To fully leverage the benefits of a Clinical Trial Management System, organizations must prioritize effective training and user adoption strategies. Comprehensive training programs should encompass all functionalities of the system, ensuring users are not only comfortable but also proficient in its use.
Studies show that 91% of organizations executing strong training programs observe a notable enhancement in adherence, with many highlighting improved operational efficiency and fewer mistakes. Ongoing support resources, such as user guides, training videos, and regular feedback sessions, can further bolster user confidence and engagement.
By promoting a culture of ongoing education and adjustment, organizations can enhance the advantages of their clinical trial management systems, resulting in better adherence outcomes and more efficient clinical trial processes. Additionally, strategies such as personalized training sessions and user-friendly interfaces can significantly enhance user adoption rates, ensuring that the system is utilized to its full potential.
Organizations should also consider assessing their current training programs against best practices to identify areas for improvement and ensure they are reaping the full financial benefits, such as the potential savings of $25K per patient noted in recent studies.
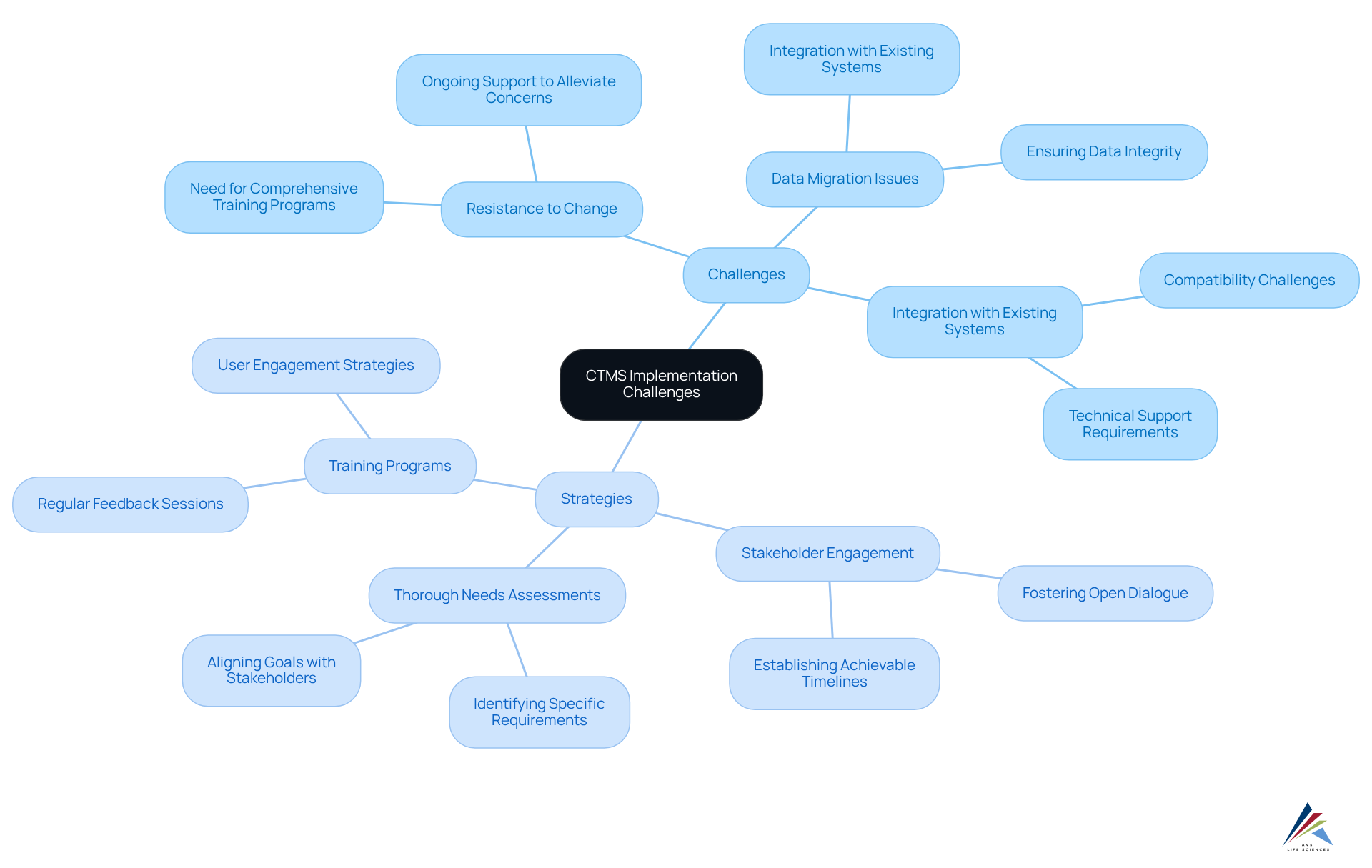
Overcoming Implementation Challenges: The Path to Successful CTMS Adoption
Implementing a CTMS clinical trial presents notable challenges, including:
- Resistance to change
- Data migration issues
- Integration with existing systems
To effectively navigate these obstacles, organizations must establish a robust implementation strategy that emphasizes:
- Stakeholder engagement
- Thorough needs assessments
Research indicates that approximately 80% of clinical studies encounter delays due to recruitment difficulties, underscoring the necessity for a well-structured approach. By establishing achievable timelines and fostering open dialogue, regulatory officers can facilitate a smoother transition.
Effective integration methods, such as those employed by GlobalCare Clinical Studies—which achieved over a 50% reduction in subject recruitment time through efficient system utilization—serve as critical examples. Addressing resistance to change is essential; organizations should implement comprehensive training programs and provide ongoing support to alleviate concerns and enhance user adoption.
By proactively tackling these challenges, regulatory officers can ensure that the clinical management system is seamlessly incorporated into existing workflows, ultimately improving efficiency and adherence in clinical studies.
Overall Impact on Clinical Trial Success: The Transformative Role of CTMS
The application of a CTMS clinical trial significantly enhances the success of clinical experiments. By improving information quality and boosting operational efficiency, the system streamlines regulatory processes, empowering organizations to conduct trials with greater effectiveness and assurance.
A notable example of this transformation is AVS Life Sciences' recent upgrade of a biotechnology GMP facility, where they assisted a leading San Francisco-based biotechnology company in transitioning from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. This upgrade not only achieved regulatory compliance but also ensured robust quality assurance, as evidenced by the client's quality assurance team's approval of AVS's documentation efforts for full traceability.
The upgrade addressed specific challenges, such as anomalies in test results due to improperly installed barcode scanner cameras, which were identified and rectified during the process. This experience underscores the critical importance of thorough testing protocols and quality management in clinical research.
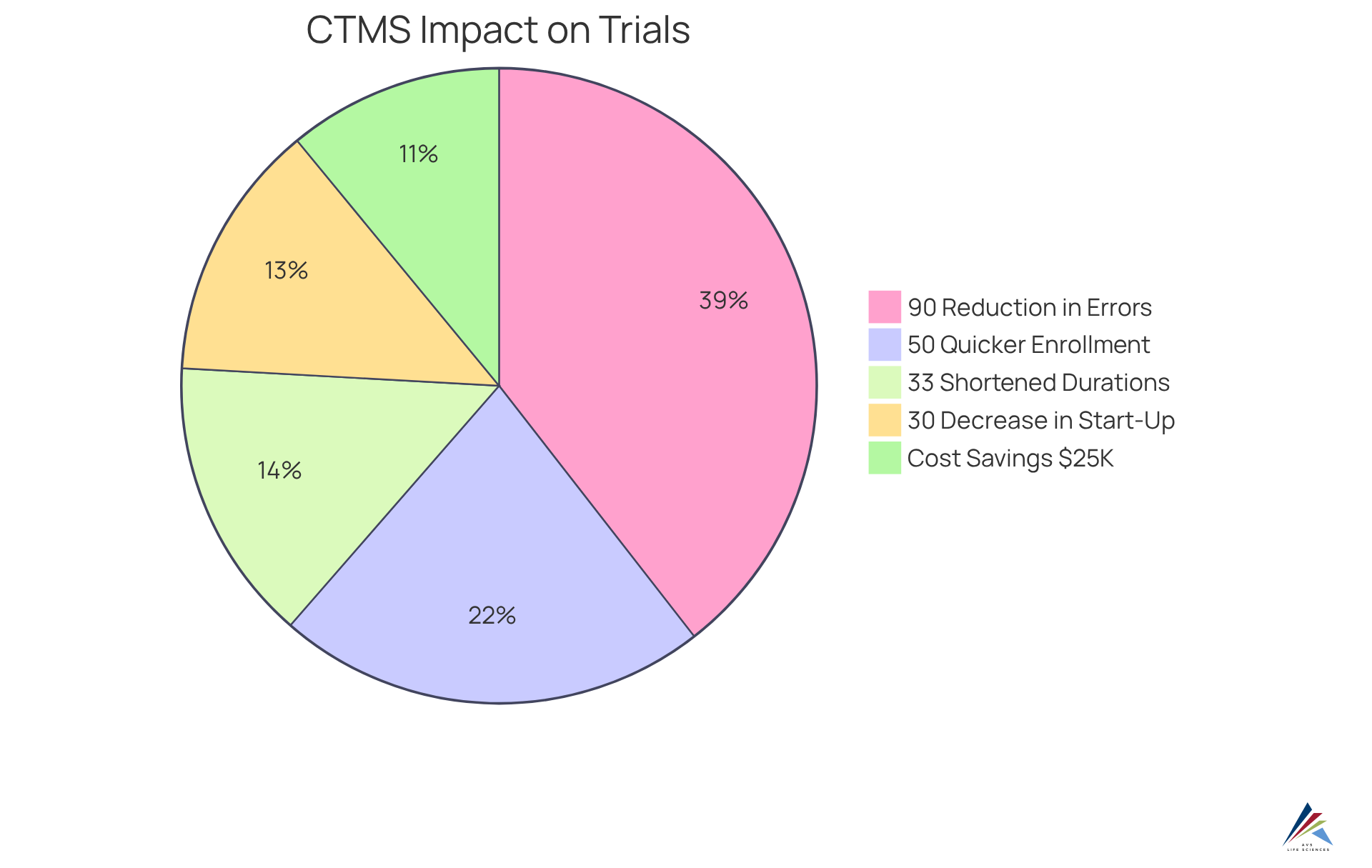
With real-time management features, the system enables strong reporting and oversight, essential for fulfilling regulatory obligations. Organizations employing the bioaccess® clinical management system, a comprehensive platform designed to enhance study management, have reported:
- A 30% decrease in study start-up times
- A 50% quicker patient enrollment rate, resulting in significant cost savings of $25,000 per patient
- A reduction in data errors by 90%
- A shortening of study durations by 33%
This emphasizes its vital role in improving overall study effectiveness. As compliance officers navigate the complexities of regulatory landscapes, the clinical trial management system emerges as an indispensable tool for achieving successful trial outcomes.
The CTMS market is projected to grow at an 11% CAGR through 2034, underscoring its increasing importance in the industry and the pivotal role of AVS Life Sciences in facilitating this growth.
Conclusion
The transformative impact of Clinical Trial Management Systems (CTMS) on compliance in clinical trials is profound and undeniable. These systems not only streamline regulatory processes but also significantly enhance data quality, improve patient management, and increase overall operational efficiency. By adopting CTMS, organizations can navigate the complexities of regulatory compliance with greater assurance, ultimately leading to more effective clinical trials.
Key benefits of CTMS include:
- Enhanced data integrity through automation
- Increased efficiency in administrative tasks
- Improved patient retention and satisfaction
The integration of real-time data access and reporting capabilities empowers compliance officers to make informed decisions swiftly. Moreover, the cost-effectiveness of CTMS reduces compliance-related penalties and optimizes resource allocation, making it a strategic asset.
In light of these insights, it is evident that the implementation of CTMS transcends a mere technological upgrade; it is a strategic necessity for organizations committed to excellence in clinical research. As the industry evolves, embracing these systems will be crucial for maintaining compliance, improving trial outcomes, and advancing the goals of the pharmaceutical and biotechnology sectors. The future of clinical trials hinges on the ability to adapt and leverage innovative solutions like CTMS, ensuring that regulatory standards are met while fostering an environment of continuous improvement and patient care.
Frequently Asked Questions
What is AVS Life Sciences known for?
AVS Life Sciences specializes in providing tailored Clinical Trial Management System (CTMS) solutions specifically designed for the pharmaceutical and biotechnology sectors, focusing on regulatory compliance and enhancing clinical research procedures.
Why are CTMS solutions important for clinical trials?
CTMS solutions are crucial for navigating regulatory environments, ensuring compliance with Good Manufacturing Practices (GMP) and Quality System Regulations (QSR), and supporting organizations in maintaining rigorous documentation and information integrity.
What is the projected market growth for CTMS by 2033?
The CTMS clinical trial market is projected to reach USD 7.9 billion by 2033, indicating a significant expansion in demand for effective clinical trial management solutions.
How do CTMS solutions benefit clinical trial management?
CTMS solutions enhance collaboration among stakeholders, improve information accuracy, reduce administrative burdens, and allow research teams to focus on patient care and timely project delivery.
What is the role of data quality and integrity in CTMS?
A major benefit of CTMS is the enhancement of information quality and integrity through centralized information management, real-time monitoring, and automation, which reduces human error and ensures compliance with regulatory standards.
What is the ALCOA framework in relation to CTMS?
The ALCOA framework stands for Attributable, Legible, Contemporaneous, Original, and Accurate, and it emphasizes the principles of information integrity in clinical studies. The expanded ALCOA+ includes additional principles such as Complete, Consistent, Enduring, and Available.
How does CTMS improve efficiency in clinical trial management?
CTMS enhances efficiency by automating tasks like scheduling, patient tracking, and document management, which alleviates administrative burdens and allows research teams to concentrate on critical activities.
Can you provide examples of companies that have benefited from CTMS?
Gilead reported a 40% reduction in regulatory deficiencies, while Pfizer experienced a 30% decrease in study initiation time after implementing clinical management systems.
What are some key features of CTMS?
Key features include automated reporting, real-time data access, and integration with platforms like Electronic Data Capture (EDC) and Laboratory Information Management Systems (LIMS), which enhance information quality and compliance.
Why is training important for using CTMS effectively?
Comprehensive training programs are essential to ensure staff proficiency in utilizing CTMS features, which maximizes the potential benefits and improves trial administration for oversight officers.
