Compare Top Clinical Research Organization Companies for Compliance Needs

Overview
This article provides a comprehensive comparison of top Clinical Research Organization (CRO) companies, with a particular focus on their compliance capabilities and service offerings. Selecting the right CRO is not merely a choice; it is a critical decision that ensures adherence to regulatory standards and significantly enhances the success of clinical trials. Detailed evaluations of leading firms such as AVS Life Sciences, ICON, IQVIA, and Parexel illustrate their unique strengths in regulatory compliance and project management. By understanding these strengths, organizations can make informed choices that align with their compliance needs and project goals.
Introduction
The landscape of clinical research is increasingly complex, with organizations facing mounting pressures to ensure compliance amidst evolving regulations. Clinical Research Organizations (CROs) play a pivotal role in this dynamic environment, offering essential services that streamline the clinical trial process and mitigate compliance risks. As sponsors seek to enhance study outcomes, the challenge lies in identifying the right CRO that not only meets regulatory standards but also aligns with specific project needs.
What key factors should organizations consider when selecting a CRO to navigate these complexities effectively? This inquiry is crucial for fostering successful partnerships and achieving compliance excellence.
Understanding Clinical Research Organizations (CROs)
Clinical research organization companies serve as essential partners in the pharmaceutical, biotechnology, and medical device sectors, delivering a comprehensive suite of services that facilitate the planning, execution, and oversight of clinical studies. Their expertise encompasses study design, site management, patient recruitment, data management, and regulatory submissions, empowering sponsors to adeptly navigate the complexities of compliance environments. This capability is critical, considering that approximately or closures due to recruitment challenges, costing sponsors between $600,000 and $8 million for each day of delay.
AVS Life Sciences exemplifies the pivotal role of CROs by ensuring adherence to ethical standards and compliance with regulatory requirements. Their extensive GXP regulatory services, including GCP audits, enable sponsors to uphold high standards while maneuvering through intricate regulatory landscapes. The COVID-19 pandemic underscored the importance of CROs in facilitating rapid vaccine development, showcasing their ability to adapt and implement decentralized research methodologies. The FDA's guidance on decentralized trials, issued in May 2023, further emphasizes the significance of local lab tests and telemedicine, which AVS Life Sciences has effectively integrated into their operational framework.
Successful case studies, such as AVS Life Sciences' transformative upgrade of a biotechnology GMP facility, highlight their commitment to quality assurance and regulatory compliance. In this instance, AVS supported a prominent San Francisco-based biotechnology firm in enhancing their manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility, completing the project on time and within budget. This collaboration enabled the client to produce medication utilizing lentivirus vector material, underscoring the critical nature of quality control and adherence in the biotech arena.
As the industry continues to evolve, the significance of clinical research organization companies in clinical studies cannot be overstated. They play a crucial role in ensuring compliance with Good Manufacturing Practices (GMP) and Quality System Regulations (QSR) while driving innovation through cutting-edge technologies. Effective communication and collaboration among stakeholders are vital for improving study outcomes, further solidifying AVS Life Sciences' position as an indispensable partner in pharmaceutical research.
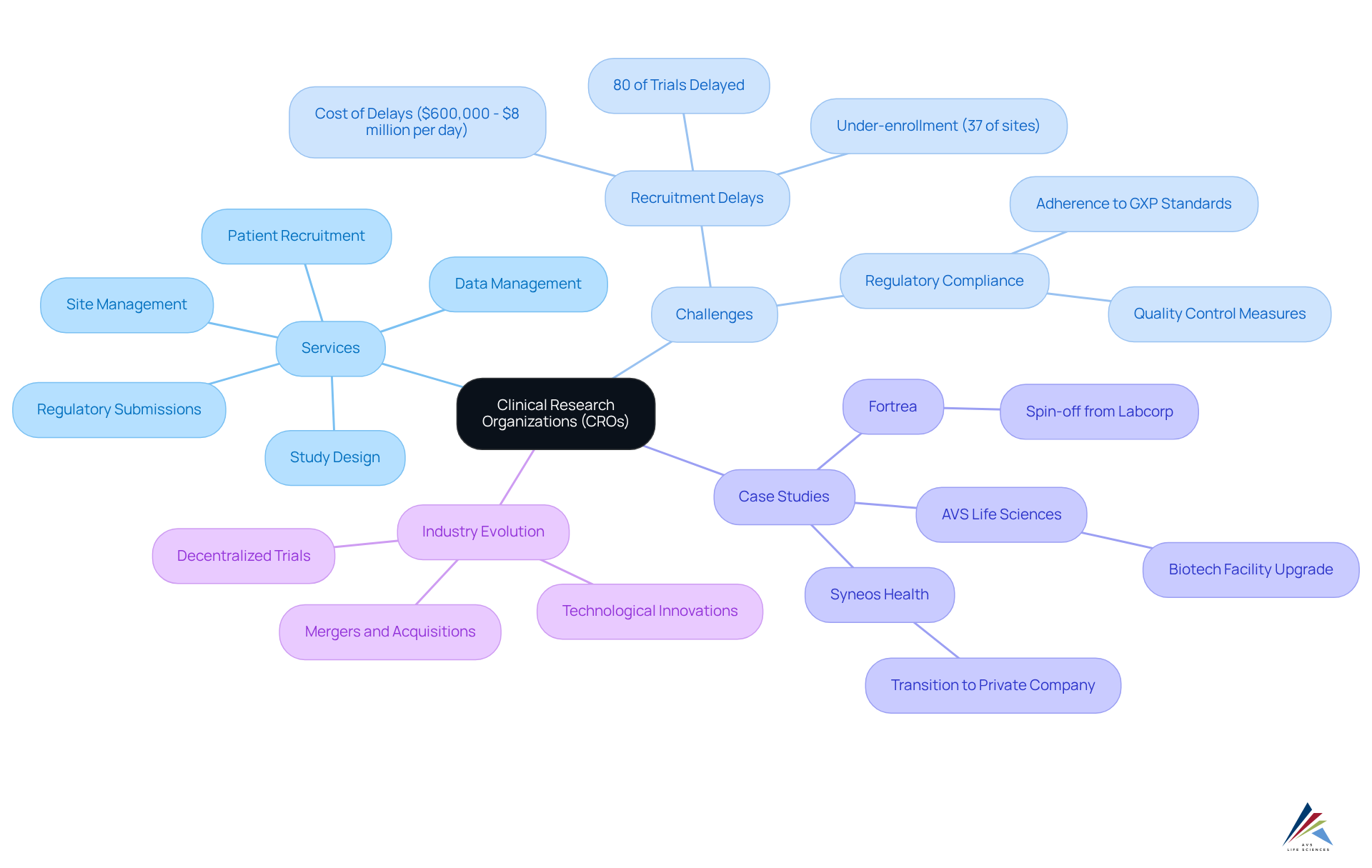
Criteria for Selecting the Right CRO
Selecting the right clinical research organization companies is vital for the success of clinical studies. Organizations must carefully consider several key factors to ensure a productive partnership:
- Therapeutic Expertise: The CRO’s experience in the specific therapeutic area relevant to the clinical study is essential. A proven track record in a particular field can provide valuable insights that enhance study design and execution.
- Regulatory Knowledge: A thorough understanding of the regulatory landscape is critical for compliance. A competent CRO should demonstrate comprehensive knowledge of regulations governing clinical studies, including Good Clinical Practice (GCP) and local requirements. AVS Life Sciences offers extensive GCP audit services, ensuring compliance for investigator sites, sponsors, and CROs. This expertise is crucial for of international proceedings, especially in regions with diverse regulatory frameworks.
- Technological Capabilities: The use of advanced technologies for data collection, oversight, and analysis significantly enhances efficiency in experimentation. Organizations should assess the CRO’s technological infrastructure, including their implementation of Electronic Data Capture (EDC) systems and Clinical Trial Management Systems (CTMS), which are vital for real-time project tracking and data integrity. AVS Life Sciences employs state-of-the-art technology to streamline processes and improve data accuracy.
- Project Coordination Skills: Effective project coordination is crucial for adhering to timelines and budgets. A CRO should possess a robust project oversight framework that ensures transparency, communication, and accountability throughout the study process. AVS Life Sciences excels in project coordination, adapting to changes and challenges to sustain project momentum and success.
- Reputation and References: Evaluating a CRO’s reputation within the industry and obtaining references from previous clients can provide insights into their reliability and performance. A strong reputation often correlates with successful project outcomes, reflecting client satisfaction and trust.
By thoroughly assessing these factors, organizations can make informed decisions when selecting clinical research organization companies, thereby enhancing the likelihood of successful outcomes in clinical trials. AVS Life Sciences stands out as a provider of quality oversight and compliance solutions for the life sciences industry, ensuring that clients receive expert quality solutions and demonstrated excellence in life sciences consulting.
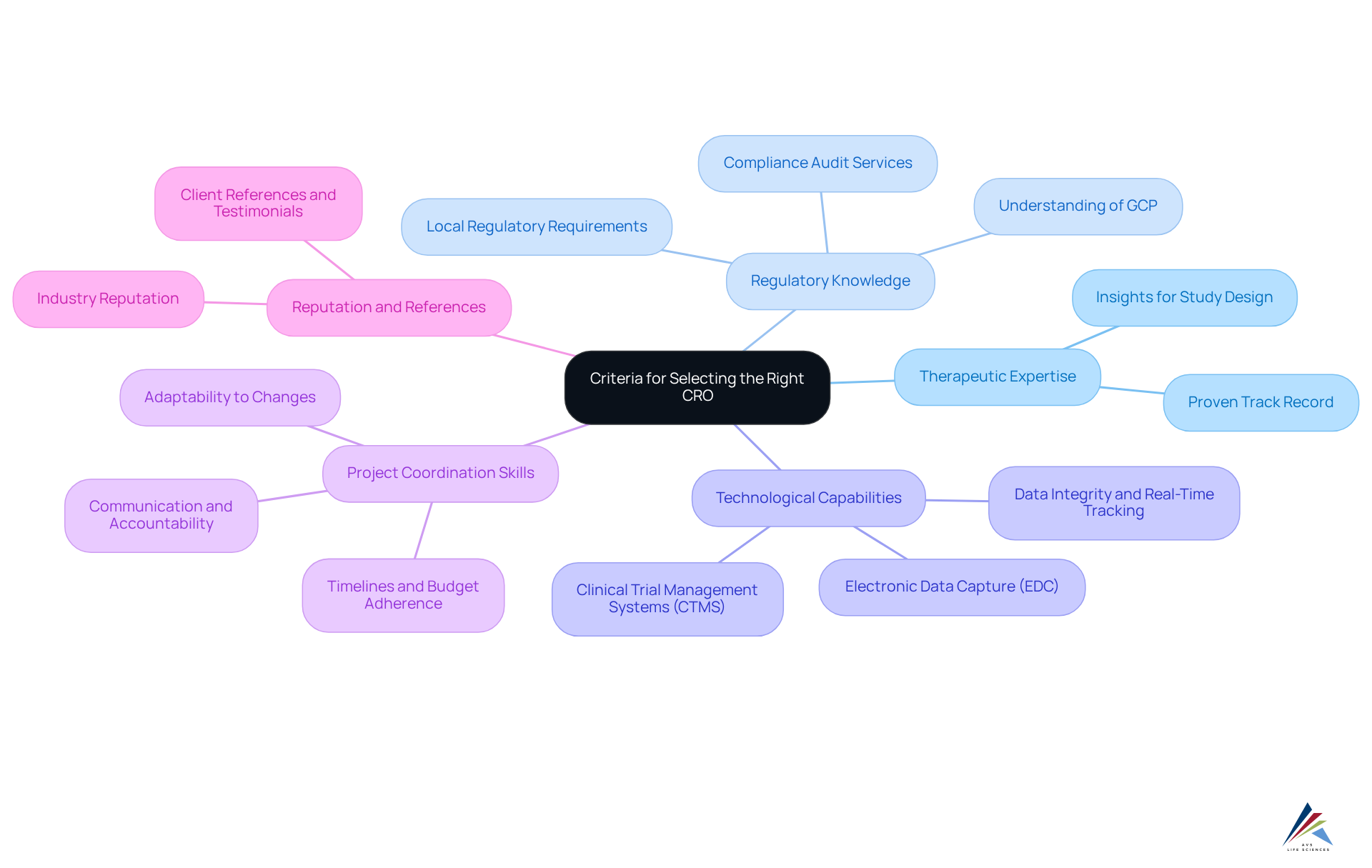
Comparative Analysis of Leading CROs
In this comparative analysis, we examine four leading clinical research organization companies: AVS Life Sciences, ICON, IQVIA, and Parexel, focusing on their compliance capabilities and service offerings.
- AVS Life Sciences: As a prominent supplier of quality oversight and adherence solutions, AVS Life Sciences is committed to ensuring that pharmaceutical firms meet strict standards. Their comprehensive approach includes robust quality management systems and a team of experts specializing in navigating complex regulatory environments. AVS Life Sciences utilizes advanced technology to improve regulatory processes, making it a valuable partner for organizations aiming to streamline operations and uphold high standards of quality.
- ICON: Renowned for its global reach and extensive therapeutic expertise, ICON excels in managing complex clinical trials. Their dedication to adherence to regulations is highlighted by strong quality management systems and a team of skilled experts. With advanced technology platforms that enhance data collection and analysis, ICON is a preferred choice for sponsors seeking efficiency and reliability. Notably, ICON has achieved a project success rate that consistently exceeds industry standards, reflecting their operational excellence.
- IQVIA: As a leader in integrating data analytics and technology within clinical research, IQVIA offers comprehensive services that span the entire drug development lifecycle. Their strong focus on rule adherence is supported by a dedicated group of specialists who ensure conformity to Good Clinical Practice (GCP) and other legal standards. IQVIA's innovative solutions, including real-world evidence studies, establish them as a progressive ally in clinical trials, with a demonstrated history of successful compliance submissions.
- Parexel: Focused on patient-centric solutions, Parexel is acknowledged for its proficiency in submission processes and adherence. Their extensive experience in navigating complex compliance environments enables clinical research organization companies to provide tailored solutions that meet the unique needs of each client. Parexel's dedication to quality is demonstrated through stringent training programs and ongoing enhancement efforts, ensuring they stay at the forefront of regulatory standards in clinical research. Parexel's success stories emphasize their capability to handle complex compliance challenges efficiently, further reinforcing their reputation in the industry.
As noted by Pete Embley, "Regulatory oversight and client support are vital at all stages of development, starting with the discovery phase and the selection of the target indication and target product profile." This highlights the significance of having a to uphold sponsor relationships and guarantee adherence.
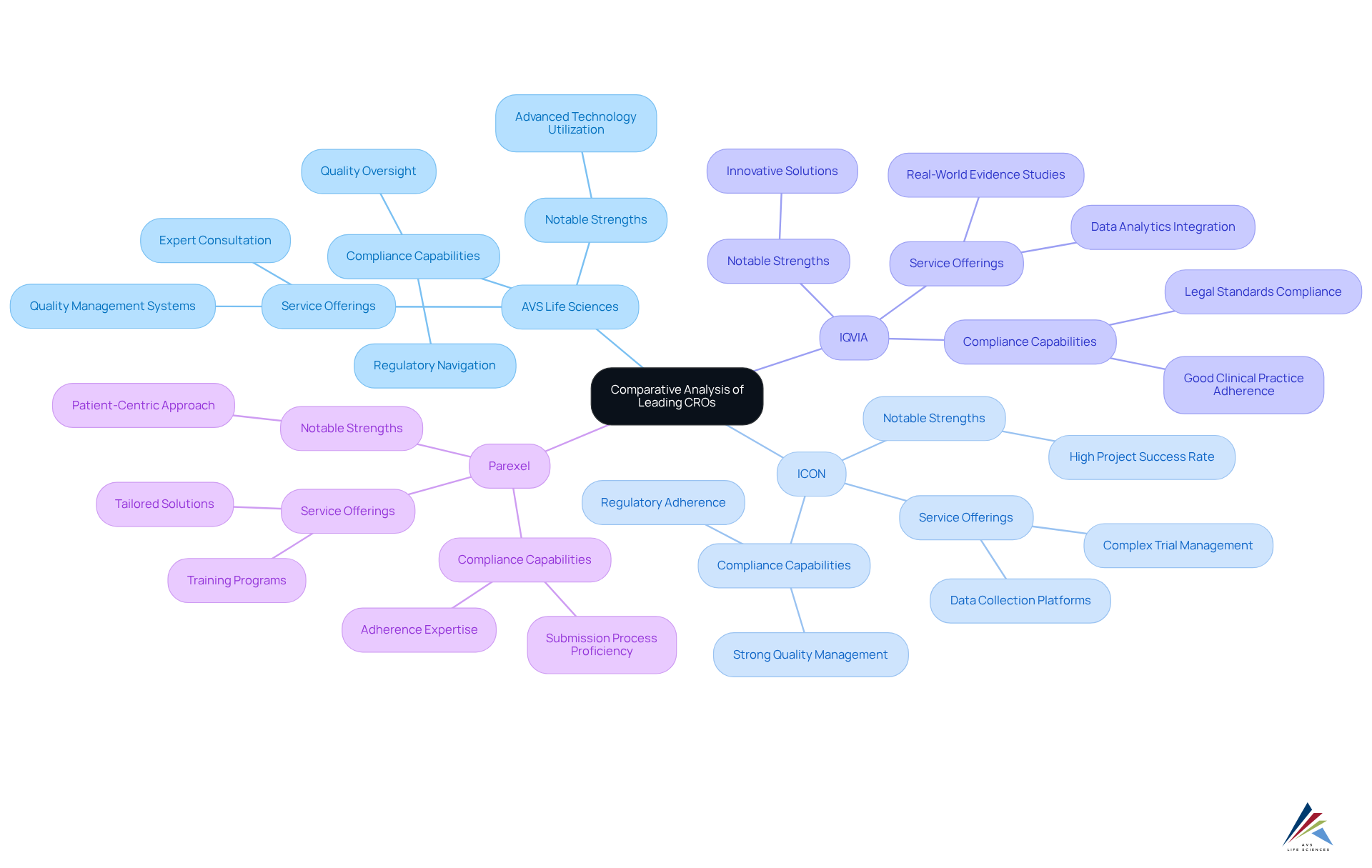
Key Takeaways for Choosing a CRO
When selecting a Contract Research Organization (CRO), it is essential to consider several key factors that will significantly impact your project's success:
- Assess Therapeutic Expertise: Ensure the CRO has a proven track record in your specific therapeutic area. This expertise enhances study design and execution, leading to more relevant endpoints and improved outcomes.
- Evaluate Compliance Knowledge: A strong understanding of the compliance landscape is crucial. Choose a CRO that demonstrates comprehensive knowledge of compliance requirements, as this can significantly mitigate risks associated with regulatory approvals.
- Consider Technological Capabilities: Opt for a CRO that employs advanced technologies for efficient data handling and analysis. The incorporation of digital tools can simplify processes and enhance data integrity, ultimately improving results.
- Prioritize Project Coordination Skills: Select clinical research organization companies that have robust project oversight practices. Effective project management ensures timely execution and adherence to budget constraints, which are vital for the success of clinical trials.
- Check Reputation and References: Investigate the CRO's reputation within the industry. Seek feedback from previous clients to assess reliability and performance, as a strong track record often correlates with successful project execution.
By focusing on these factors, organizations can make informed decisions when selecting a CRO that aligns with their compliance needs and project goals.

Conclusion
Clinical research organizations (CROs) play a pivotal role in clinical studies, offering essential services that ensure compliance and facilitate the successful execution of trials. Their expertise not only navigates complex regulatory environments but also drives innovation and efficiency in drug development. The significance of CROs, particularly in light of recent global challenges such as the COVID-19 pandemic, underscores their adaptability and crucial role in advancing medical research.
When selecting the right CRO, key factors to consider include:
- Therapeutic expertise
- Regulatory knowledge
- Technological capabilities
- Project coordination skills
- A solid reputation
Each of these elements is vital in determining the effectiveness and reliability of a CRO partnership. Companies like AVS Life Sciences, ICON, IQVIA, and Parexel exemplify the diverse strengths and specialized services available, highlighting the importance of thorough evaluation when choosing a CRO.
As the landscape of clinical research evolves, the necessity for strategic partnerships with competent CROs becomes increasingly vital. Organizations must prioritize informed decision-making based on these outlined criteria to enhance the likelihood of successful clinical trials. By doing so, they not only improve compliance but also contribute to the advancement of healthcare solutions that can benefit society as a whole.
Frequently Asked Questions
What are Clinical Research Organizations (CROs)?
Clinical Research Organizations (CROs) are companies that provide essential services to the pharmaceutical, biotechnology, and medical device sectors, facilitating the planning, execution, and oversight of clinical studies.
What services do CROs offer?
CROs offer a comprehensive suite of services including study design, site management, patient recruitment, data management, and regulatory submissions.
Why are CROs important in clinical trials?
CROs are crucial because they help sponsors navigate complex compliance environments, which is vital since approximately 80% of clinical trials face delays or closures due to recruitment challenges.
What are the financial implications of delays in clinical trials?
Delays in clinical trials can cost sponsors between $600,000 and $8 million for each day of delay.
How did the COVID-19 pandemic affect the role of CROs?
The COVID-19 pandemic highlighted the importance of CROs in facilitating rapid vaccine development and showcased their ability to adapt by implementing decentralized research methodologies.
What guidance did the FDA provide regarding decentralized trials?
In May 2023, the FDA issued guidance on decentralized trials, emphasizing the importance of local lab tests and telemedicine, which CROs like AVS Life Sciences have integrated into their operations.
Can you provide an example of a successful CRO project?
A successful project involved AVS Life Sciences upgrading a biotechnology GMP facility from a Biosafety Level 1 to a Level 2 GMP facility for a San Francisco-based biotechnology firm, which was completed on time and within budget.
What is the significance of quality control in the biotech industry?
Quality control is critical in the biotech sector, as demonstrated by AVS Life Sciences' collaboration, which enabled the client to produce medication using lentivirus vector material, ensuring adherence to regulatory standards.
How do CROs contribute to innovation in clinical research?
CROs drive innovation by ensuring compliance with Good Manufacturing Practices (GMP) and Quality System Regulations (QSR) while leveraging cutting-edge technologies.
What is essential for improving study outcomes in clinical research?
Effective communication and collaboration among stakeholders are vital for improving study outcomes in clinical research.
