Master CAPA Pharma: Essential Best Practices for Compliance Officers

Overview
This article delves into the essential best practices that compliance officers must master regarding Corrective and Preventive Actions (CAPA) within the pharmaceutical industry. It underscores the necessity of a structured CAPA process, highlighting how the integration of technology and the adoption of best practices can significantly enhance compliance. By fostering a culture of continuous improvement, organizations can ensure product quality and regulatory adherence.
Compliance challenges are prevalent in the industry; however, implementing these strategies can lead to effective solutions. Compliance officers are encouraged to engage with these practices actively, ensuring they remain at the forefront of regulatory standards.
Introduction
In the intricate landscape of the pharmaceutical industry, the implementation of Corrective and Preventive Actions (CAPA) serves as a critical pillar for ensuring compliance and quality. As regulatory standards evolve and the stakes heighten, compliance officers face complex challenges that demand not only navigation but also the fostering of a culture of continuous improvement. This article delves into essential best practices for CAPA management, offering insights that empower compliance professionals to enhance their strategies and effectively mitigate risks.
How can organizations leverage these practices to not only meet regulatory demands but also drive innovation and excellence in their operations?
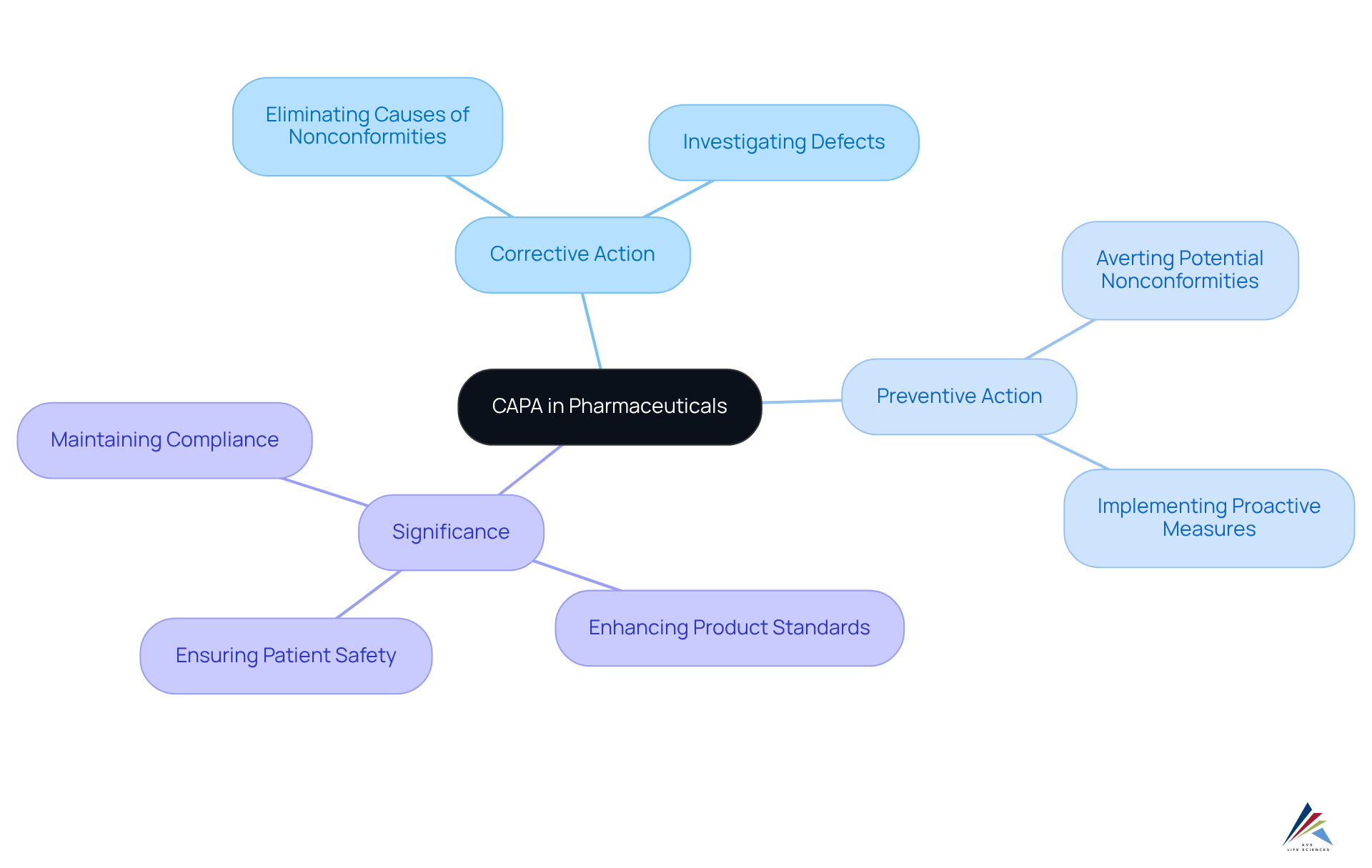
Define CAPA: Core Concepts and Importance in Pharmaceuticals
Corrective and Preventive Actions are systematic processes employed within the pharmaceutical sector to identify, investigate, and rectify nonconformities in products and procedures. These actions are essential for ensuring compliance with regulatory standards such as Good Manufacturing Practices (GMP) and FDA regulations. The core concepts of CAPA encompass:
- Corrective Action: Steps taken to eliminate the causes of existing nonconformities or defects.
- Preventive Action: Measures implemented to avert potential nonconformities from arising in the future.
The significance of corrective actions and preventive measures lies in their ability to enhance product standards, ensure patient safety, and maintain compliance with regulatory mandates. A robust capa pharma system not only addresses immediate issues but also fosters a culture of continuous improvement within organizations, ultimately leading to better outcomes in the pharmaceutical industry. By integrating comprehensive management practices—including effective documentation, adherence to Standard Operating Procedures (SOPs), and addressing Data Integrity Deviations—AVS Life Sciences empowers organizations to achieve excellence in .
Outline the CAPA Process: Step-by-Step Implementation Guide
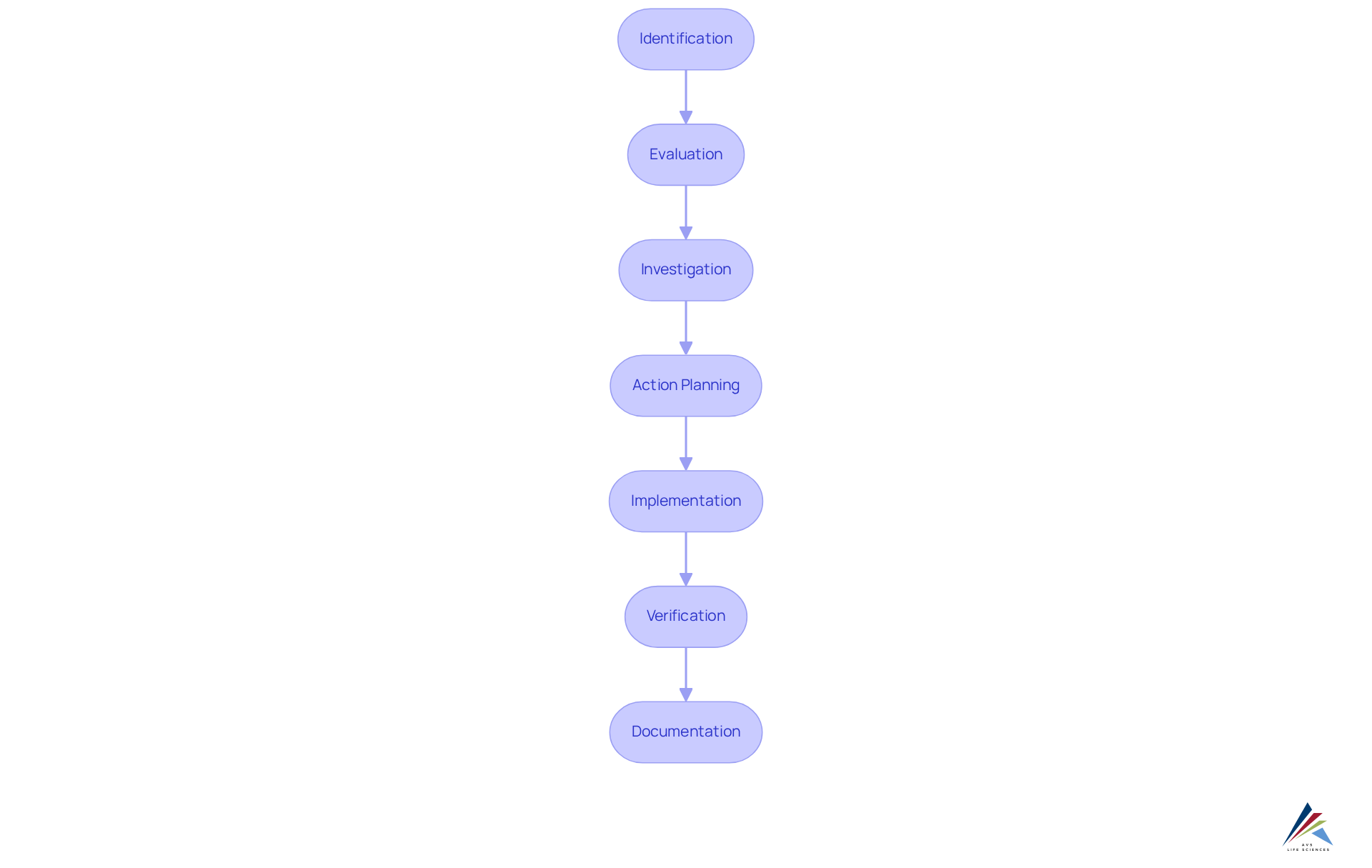
The CAPA process is essential for maintaining compliance and can be broken down into several key steps:
- Identification: Recognize and document the issue or nonconformity.
- Evaluation: Assess the seriousness and effect of the issue on product standards and adherence.
- Investigation: Conduct a thorough investigation to determine the root cause of the problem.
- Action Planning: Develop a corrective action plan that outlines the steps needed to address the issue.
- Implementation: Execute the corrective action plan and ensure all stakeholders are informed.
- Verification: Monitor the effectiveness of the corrective actions taken to ensure the issue does not recur.
- Documentation: Keep detailed records of the corrective and preventive actions, including findings, actions taken, and outcomes.
Adopting this organized method ensures that capa pharma personnel can efficiently oversee corrective and preventive actions while maintaining elevated levels of quality and standards. This structured approach not only mitigates risks but also fosters a culture of within the organization.
Implement Best Practices: Strategies for Effective CAPA Management
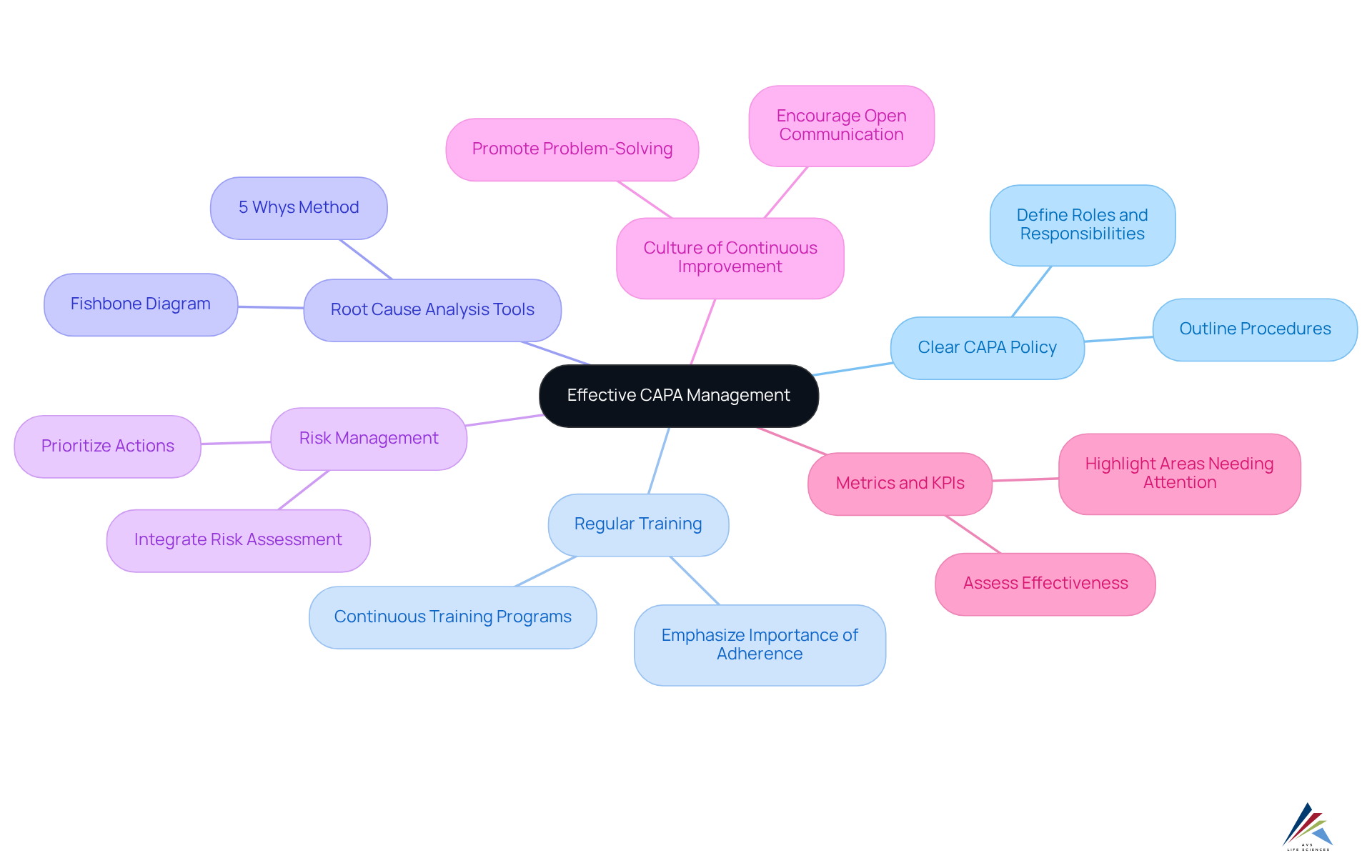
To ensure effective capa pharma management, compliance officers must adopt best practices that address and enhance organizational quality.
- Establish a Clear CAPA Policy: Develop and communicate a comprehensive CAPA policy that outlines roles, responsibilities, and procedures. This foundational step sets the stage for accountability and clarity within the organization.
- Conduct Regular Training: Offer continuous training for personnel engaged in corrective and preventive action. This ensures that team members comprehend their responsibilities and the significance of adherence, fostering a knowledgeable workforce.
- Utilize Root Cause Analysis Tools: Implement tools such as the 5 Whys or Fishbone Diagram to facilitate thorough investigations and identify root causes. By employing these methods, compliance officers can effectively address underlying issues.
- Incorporate Risk Management: Integrate risk assessment into the corrective and preventive action process. This allows for prioritization of actions based on potential impact, ensuring that resources are allocated efficiently.
- Foster a Culture of Continuous Improvement: Encourage open communication and feedback to identify areas for improvement. This proactive approach promotes problem-solving and innovation within the organization.
- Leverage Metrics and KPIs: Use key performance indicators to assess the effectiveness of corrective and preventive actions. This drives accountability and highlights areas needing attention.
By adopting these best practices, compliance personnel at capa pharma can significantly enhance their corrective and preventive action systems, contributing to a culture of quality and adherence within their organizations.
Leverage Technology: Enhancing CAPA Efficiency and Compliance
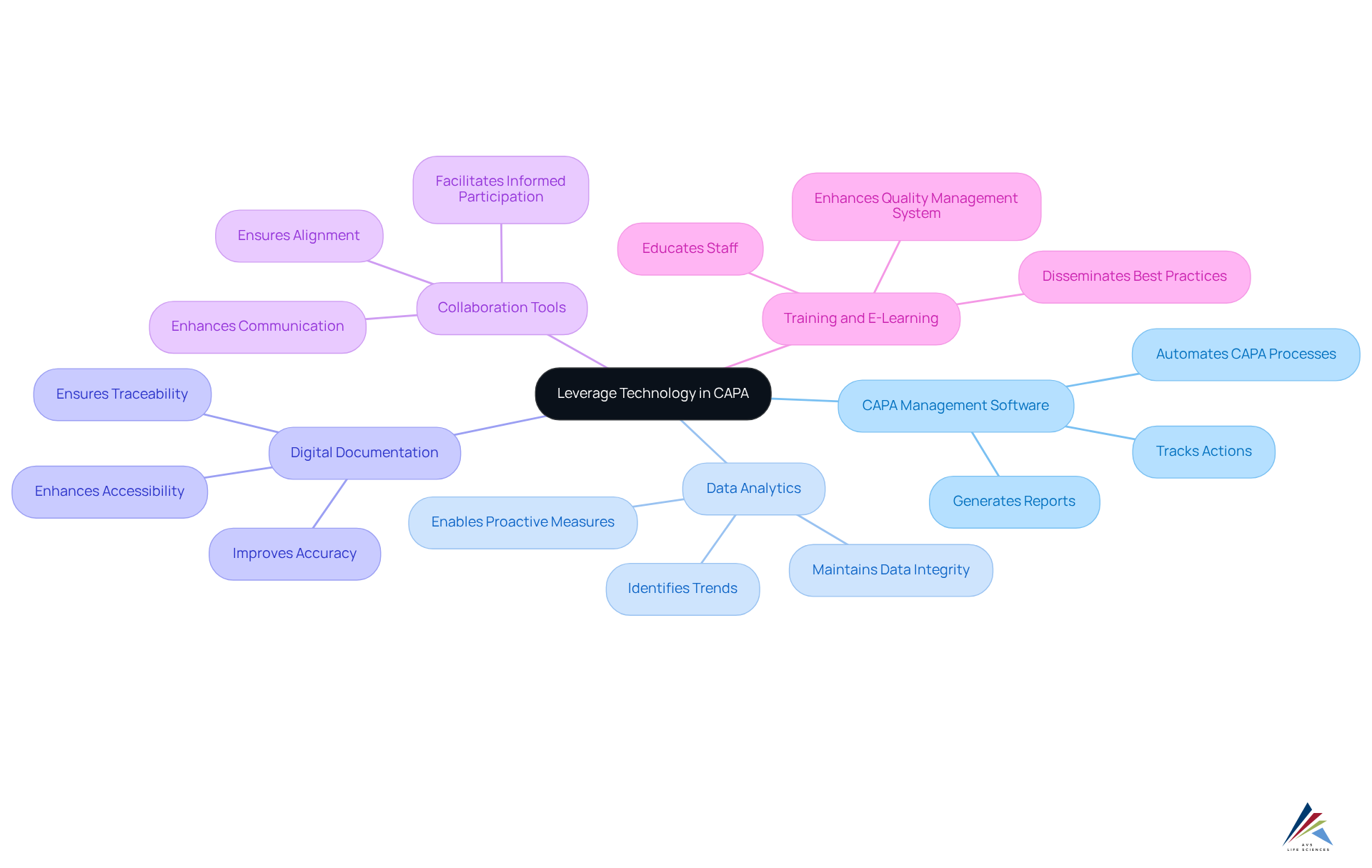
Integrating technology into corrective and preventive action management significantly enhances efficiency and adherence, particularly within the highly regulated life sciences sector. Consider these strategies to leverage technology effectively:
- CAPA Management Software: Implement specialized software that automates CAPA processes, tracks actions, and generates reports to streamline workflows, ensuring adherence to FDA regulations and GXP standards.
- Data Analytics: Utilize data analytics tools to identify trends and patterns in nonconformities, enabling proactive measures to prevent future issues. This approach is crucial for maintaining data integrity and compliance with regulations.
- Digital Documentation: Transition to electronic documentation systems to enhance accessibility, accuracy, and traceability of corrective and preventive action records, aligning with excellent documentation practices and standard operating procedures (SOPs).
- Collaboration Tools: Utilize collaboration platforms to enhance communication among team members engaged in the corrective and preventive action process. This ensures alignment and informed participation, essential for effective project management and regulatory adherence.
- Training and E-Learning: Implement e-learning platforms to educate staff on corrective and preventive action procedures, facilitating the dissemination of best practices and regulatory requirements, thus enhancing the overall quality management system.
By leveraging technology, compliance officers can enhance the effectiveness of their compliance management by improving their CAPA pharma systems, reducing manual errors, and ensuring a more streamlined approach. This ultimately supports AVS Life Sciences' commitment to delivering and validation.
Conclusion
The essence of effective Corrective and Preventive Actions (CAPA) in the pharmaceutical industry is paramount. By systematically addressing nonconformities, organizations not only comply with regulatory standards but also significantly enhance product quality and ensure patient safety. A well-implemented CAPA process cultivates a culture of continuous improvement, establishing it as a cornerstone of operational excellence in pharmaceuticals.
Key strategies for successful CAPA management encompass:
- The establishment of clear policies
- Regular training
- The utilization of root cause analysis tools
- The integration of risk management practices
Leveraging technology, such as specialized CAPA management software and data analytics, streamlines processes and bolsters compliance. Each of these components contributes to a robust framework that empowers compliance officers to effectively oversee corrective and preventive actions.
Ultimately, the commitment to mastering CAPA practices is essential for any organization aspiring to thrive in the highly regulated pharmaceutical landscape. By embracing these best practices and technological advancements, compliance officers can not only mitigate risks but also drive innovation and quality improvements. The significance of CAPA transcends mere compliance; it embodies a proactive approach to ensuring the highest standards of safety and efficacy in drug development and manufacturing.
Frequently Asked Questions
What does CAPA stand for in the pharmaceutical industry?
CAPA stands for Corrective and Preventive Actions, which are systematic processes used to identify, investigate, and rectify nonconformities in products and procedures.
What are the core concepts of CAPA?
The core concepts of CAPA include Corrective Action, which involves steps taken to eliminate the causes of existing nonconformities or defects, and Preventive Action, which includes measures implemented to prevent potential nonconformities from arising in the future.
Why are CAPA processes important in pharmaceuticals?
CAPA processes are crucial for enhancing product standards, ensuring patient safety, and maintaining compliance with regulatory mandates like Good Manufacturing Practices (GMP) and FDA regulations.
How does a robust CAPA system benefit pharmaceutical organizations?
A robust CAPA system addresses immediate issues and fosters a culture of continuous improvement, leading to better outcomes in the pharmaceutical industry.
What management practices are integrated into an effective CAPA system?
Effective management practices include comprehensive documentation, adherence to Standard Operating Procedures (SOPs), and addressing Data Integrity Deviations.
How does AVS Life Sciences support organizations in achieving regulatory compliance?
AVS Life Sciences empowers organizations to achieve excellence in regulatory compliance and assurance by implementing comprehensive management practices related to CAPA.
