Master Pharmaceutical Compliance: 4 Steps for Success

Overview
Mastering pharmaceutical compliance requires a strategic approach centered on four essential steps:
- Understanding compliance fundamentals
- Identifying key regulatory frameworks
- Developing a comprehensive compliance program
- Establishing continuous monitoring and auditing processes
These steps are critical for ensuring adherence to regulations such as GMP and QSR, significantly reducing the risk of non-compliance. Moreover, they foster a culture of accountability within organizations, ultimately safeguarding product quality and consumer safety. By implementing these strategies, organizations can not only navigate the complexities of compliance but also enhance their operational integrity and trustworthiness in the marketplace.
Introduction
Navigating the complex landscape of pharmaceutical compliance is critical for organizations aiming to ensure the safety and efficacy of their products. In an ever-evolving regulatory environment, understanding foundational elements such as:
- Good Manufacturing Practices (GMP)
- Quality System Regulations (QSR)
- ISO Standards
is not merely beneficial—it is essential. As compliance requirements grow increasingly stringent, a pressing challenge emerges: how can companies effectively implement and sustain a comprehensive compliance program that not only meets regulatory standards but also fosters a culture of accountability and continuous improvement? This guide explores four pivotal steps designed to empower organizations to master pharmaceutical compliance and safeguard their operations against potential pitfalls.
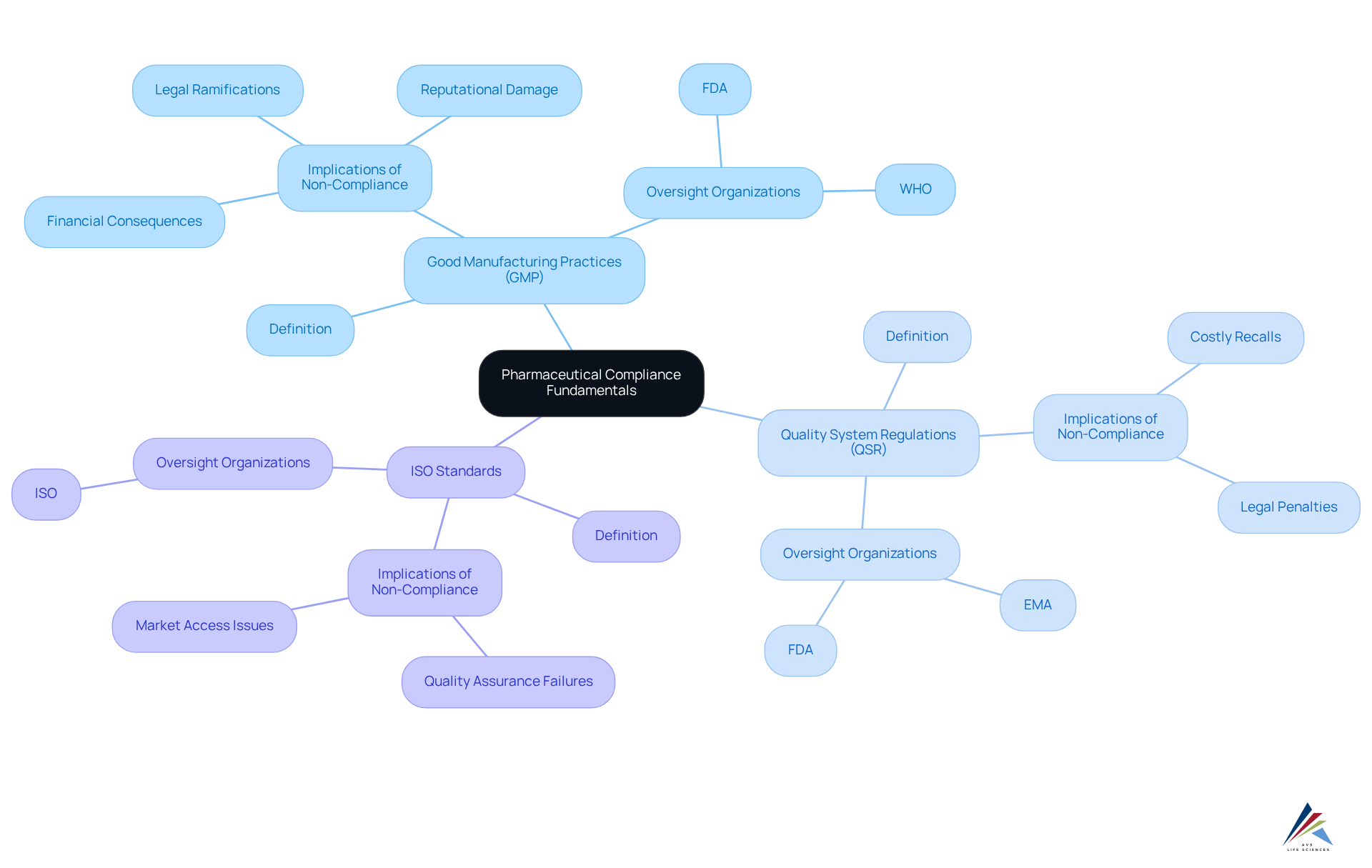
Understand Pharmaceutical Compliance Fundamentals
Pharmaceutical compliance encompasses the laws, regulations, and guidelines that govern the development, manufacturing, and marketing of pharmaceutical products. Understanding these key components is essential for navigating the in the industry.
Good Manufacturing Practices (GMP) are critical regulations that ensure products are consistently produced and controlled in accordance with quality standards, safeguarding consumer safety and product efficacy. The integration of digital tools and new technologies within GMP guidelines enhances pharmaceutical compliance and boosts operational efficiency, allowing organizations to adapt to evolving industry demands.
Quality System Regulations (QSR) oversee the quality management systems for medical devices, underscoring the necessity of maintaining stringent quality criteria throughout the product lifecycle. Non-compliance with QSR can result in severe legal and financial ramifications, including costly recalls and reputational damage. A notable instance involved a medical device manufacturer facing substantial penalties due to inadequate quality controls, emphasizing the critical need for robust adherence practices.
ISO Standards represent international benchmarks that ensure quality, safety, and efficiency in products and services. These standards complement GMP and QSR by providing a structured framework for continuous improvement and risk management.
Familiarity with these fundamentals is vital for understanding how pharmaceutical compliance impacts every phase of the pharmaceutical lifecycle, from research and development to post-market surveillance. As Peter Drucker aptly noted, 'If you can’t measure it, you can’t improve it,' highlighting the importance of data-driven approaches in compliance management.
Action Steps:
- Review the definitions of GMP, QSR, and ISO standards.
- Familiarize yourself with the oversight organizations involved, such as the FDA and EMA.
- Comprehend the implications of non-compliance, including potential legal and financial repercussions.
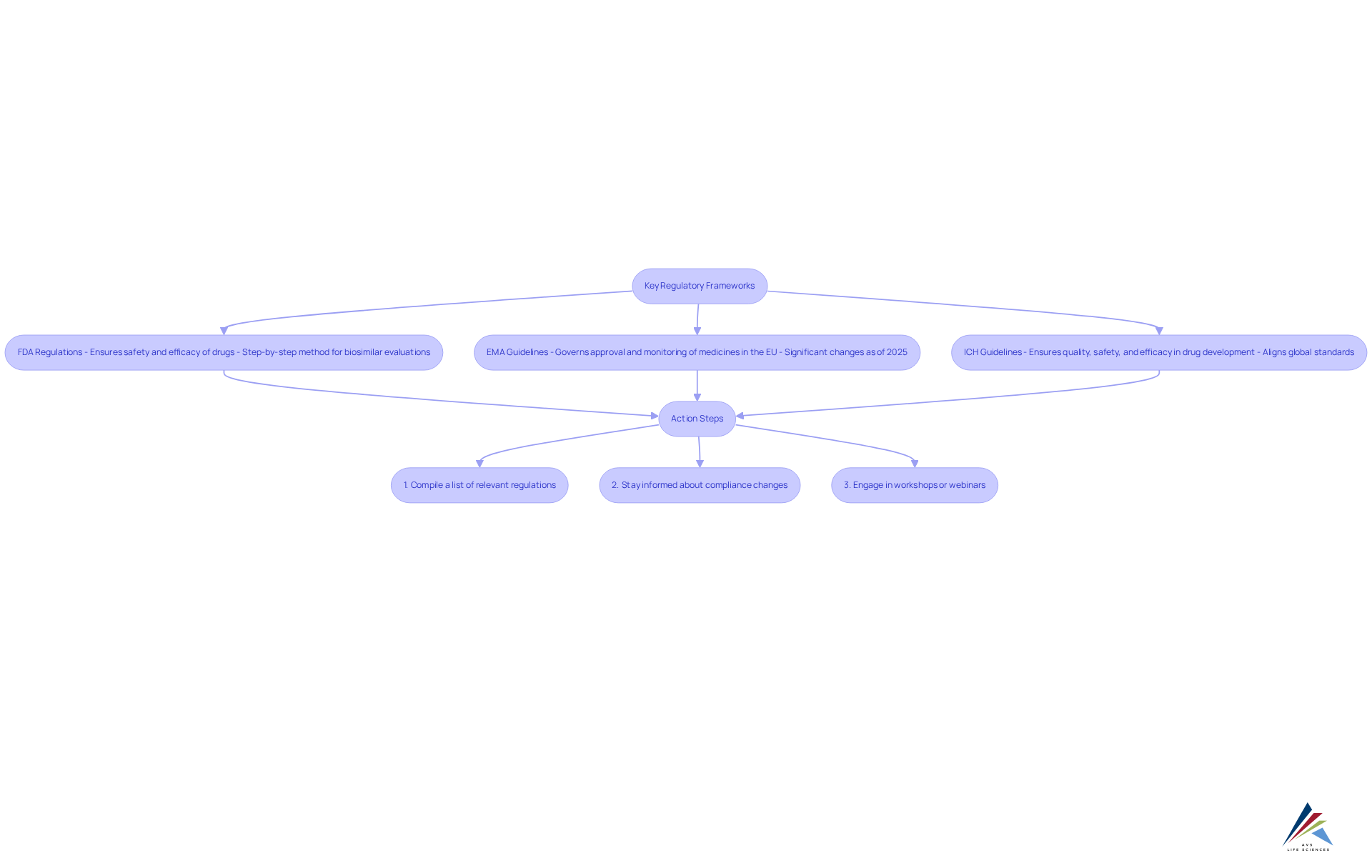
Identify Key Regulatory Frameworks and Standards
Navigating pharmaceutical compliance requires a thorough understanding of the essential regulatory frameworks and guidelines that govern your organization. These include:
- FDA Regulations: The FDA enforces stringent regulations to ensure the safety and efficacy of drugs and medical devices. This encompasses a step-by-step method for biosimilar evaluations, emphasizing analytical similarity and pharmacokinetics, which is essential for upholding high safety measures.
- European Medicines Agency (EMA) Guidelines: The EMA's guidelines, which are subject to updates, govern the approval and monitoring of medicines within the European Union. As of 2025, significant changes have been made to enhance the clarity and efficiency of the approval process, including the introduction of new scientific guidelines for evaluating new active substances (NAS).
- ICH Guidelines: The International Council for Harmonisation (ICH) provides comprehensive guidelines that guarantee quality, safety, and efficacy in drug development. These guidelines are essential for aligning global standards of pharmaceutical compliance and facilitating smoother market access.
To ensure compliance with these frameworks, consider the following action steps:
- Compile a list of relevant regulations applicable to your organization, focusing on both FDA and EMA requirements.
- Stay informed about compliance changes by subscribing to industry newsletters and updates from governing agencies.
- Engage in workshops or webinars focused on industry standards to enhance your knowledge and remain informed about market trends.
Develop and Implement a Comprehensive Compliance Program
A robust program for pharmaceutical compliance is essential for ensuring effectiveness and adherence to regulatory standards. It must include several key elements:
- Written Policies and Procedures: Establish clear, well-documented policies that articulate compliance expectations and procedures. These documents serve as a foundational reference for all employees, ensuring everyone understands their responsibilities and the standards they must uphold.
- Training and Education: Implement regular training sessions to keep employees informed about regulatory requirements and their specific roles in maintaining them. Given that human error accounts for 54% of adherence failures, continuous education is critical. By 2025, 60% of pharmaceutical companies are projected to adopt digital learning platforms to enhance training programs, reflecting the industry's commitment to continuous improvement. Furthermore, with 45% of companies expected to implement AI tools for regulatory tasks by 2025, leveraging technology can significantly enhance training efficiency and management.
- Risk Assessment: Conduct periodic risk evaluations to identify potential regulatory vulnerabilities and develop strategies to mitigate them. This proactive approach not only aids in upholding regulations but also . The costs associated with non-adherence can be substantial, with an average of $14.8 million per infraction anticipated in 2025, highlighting the financial necessity of a robust pharmaceutical compliance program.
- Computer System Validation (CSV): Integrate a comprehensive CSV process that follows the V-Model outlined in the Good Automated Manufacturing Practices (GAMP) 5 Guide. This includes stages such as planning, defining User Requirement Specifications (URS), design specifications, and rigorous testing phases (IQ, OQ, PQ) to ensure that systems operate as intended and meet regulatory standards. The expertise of 'AVS Life Sciences' in this area, demonstrated through successful case studies, highlights the importance of thorough documentation and quality assurance in achieving adherence.
Action Steps:
- Draft a thorough regulatory manual that encompasses all pertinent policies and procedures.
- Schedule regular training sessions, ensuring attendance is documented to track employee engagement.
- Establish a risk assessment framework to periodically evaluate regulatory risks and adjust strategies accordingly.
- Conduct regular audits to ensure ongoing adherence and enhancement.

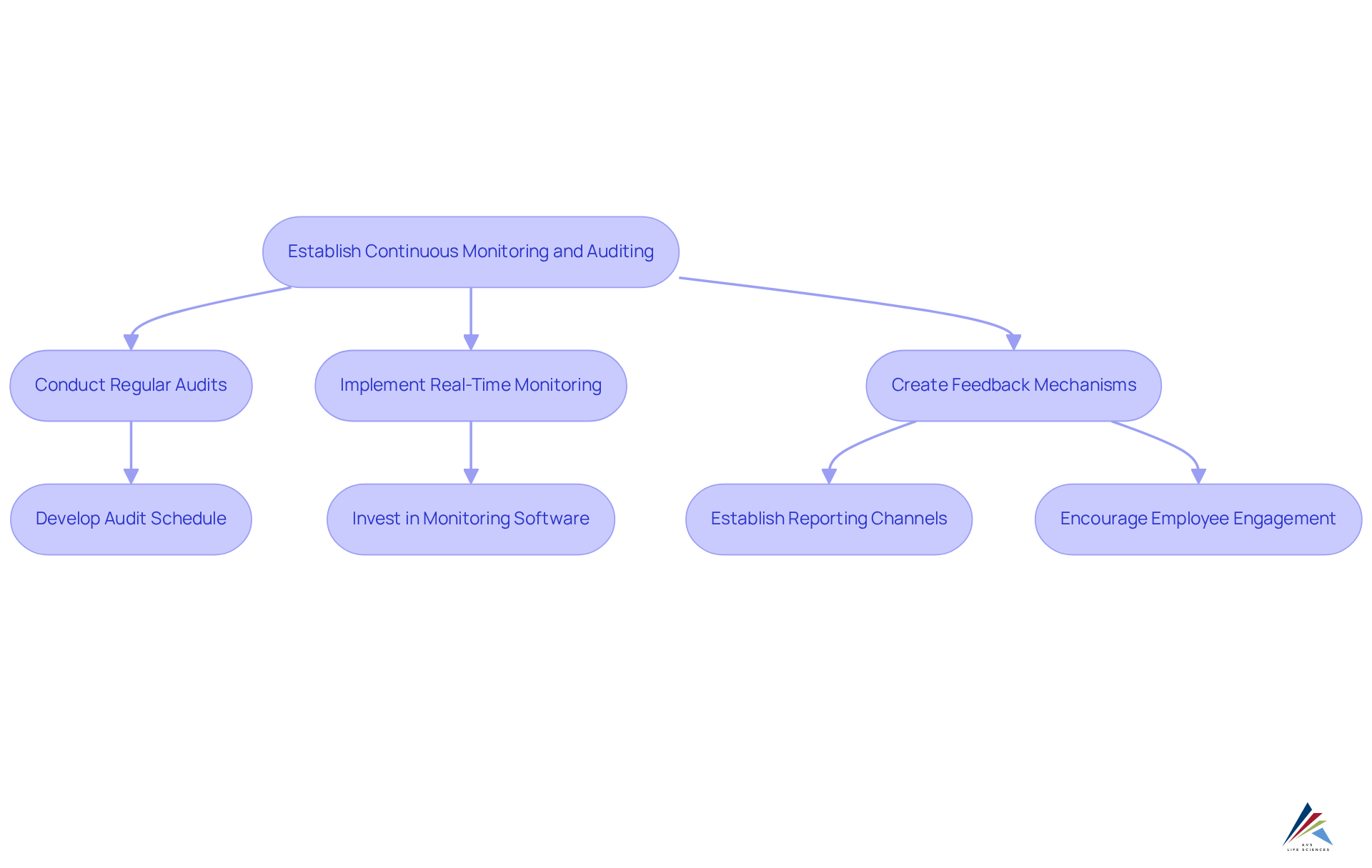
Establish Continuous Monitoring and Auditing Processes
Establishing ongoing monitoring and auditing procedures is crucial for achieving pharmaceutical compliance in an effective regulatory program in the pharmaceutical sector. Regular audits serve as a foundational practice, enabling organizations to evaluate their pharmaceutical compliance with established policies and regulatory requirements. These audits not only uncover adherence gaps but also foster a culture of responsibility among employees.
Furthermore, implementing real-time monitoring systems significantly enhances pharmaceutical compliance and regulatory management. Organizations that adopt these systems for pharmaceutical compliance have reported a 30% faster incident detection rate, facilitating immediate corrective actions when necessary. The integration of has also led to a 25% increase in overall adherence scores, significantly improving pharmaceutical compliance and resulting in a remarkable 40% reduction in remediation time.
Additionally, establishing robust feedback mechanisms is vital. Creating channels for employees to report adherence concerns or propose improvements fosters transparency, encourages proactive engagement, and helps identify potential issues before they escalate.
To advance these initiatives, consider the following action steps:
- Develop a comprehensive audit schedule that outlines the frequency and methodology of audits.
- Invest in advanced oversight monitoring software to effectively track key performance indicators and regulatory metrics.
- Cultivate a culture of transparency where employees feel empowered to report issues without fear of repercussions, thereby enhancing the effectiveness of pharmaceutical compliance.
Conclusion
Mastering pharmaceutical compliance is not merely a regulatory obligation; it is a critical component of safeguarding public health and ensuring the integrity of pharmaceutical products. By grasping the core principles of compliance—such as Good Manufacturing Practices (GMP), Quality System Regulations (QSR), and ISO standards—organizations can adeptly navigate the complexities of the industry and uphold high-quality standards throughout the product lifecycle.
This article underscores essential steps for achieving compliance, including:
- The development of a comprehensive compliance program
- The implementation of continuous monitoring and auditing processes
- The necessity of staying informed about regulatory changes
By establishing clear policies, providing ongoing training, and conducting regular audits, organizations can significantly mitigate the risk of non-compliance while cultivating a culture of accountability and transparency.
The significance of pharmaceutical compliance cannot be overstated. As the industry evolves, the adoption of innovative technologies and proactive strategies will be vital for maintaining compliance and enhancing operational efficiency. Organizations are urged to prioritize these practices not only to satisfy regulatory demands but also to foster trust with consumers and stakeholders, thereby ensuring a safer and more effective pharmaceutical landscape.
Frequently Asked Questions
What is pharmaceutical compliance?
Pharmaceutical compliance encompasses the laws, regulations, and guidelines that govern the development, manufacturing, and marketing of pharmaceutical products.
Why are Good Manufacturing Practices (GMP) important?
GMP regulations ensure that products are consistently produced and controlled according to quality standards, which safeguards consumer safety and product efficacy.
How do digital tools and new technologies impact GMP?
The integration of digital tools and new technologies within GMP guidelines enhances pharmaceutical compliance and boosts operational efficiency, allowing organizations to adapt to evolving industry demands.
What are Quality System Regulations (QSR)?
QSR oversee the quality management systems for medical devices, ensuring that stringent quality criteria are maintained throughout the product lifecycle.
What are the consequences of non-compliance with QSR?
Non-compliance with QSR can lead to severe legal and financial repercussions, including costly recalls and reputational damage.
What role do ISO Standards play in pharmaceutical compliance?
ISO Standards provide international benchmarks that ensure quality, safety, and efficiency in products and services, complementing GMP and QSR by offering a structured framework for continuous improvement and risk management.
Why is familiarity with pharmaceutical compliance fundamentals important?
Understanding these fundamentals is vital for recognizing how pharmaceutical compliance impacts every phase of the pharmaceutical lifecycle, from research and development to post-market surveillance.
What are some action steps to improve understanding of pharmaceutical compliance?
Key action steps include reviewing the definitions of GMP, QSR, and ISO standards, familiarizing oneself with oversight organizations like the FDA and EMA, and comprehending the implications of non-compliance, including potential legal and financial repercussions.
